Unit Two
Day 13: Noncovalent Interactions
As you work through this section, if you find that you need a bit more background material to help you understand the topics at hand, you can consult “Chemistry: The Molecular Science” (5th ed. Moore and Stanitski) Chapter 7-5, 7-6, 9-1 through 9-3a, 9-5, and/or Chapter 7.1-7.4 in the Additional Reading Materials section.
D13.1 Forces Between Molecules
Based on the simulation, write in your notebook a description of the differences in position and movement of atomic-level particles in solids, liquids, and gases.
Whether a substance is a solid, liquid, or gas at room temperature (or any other temperature) depends on the strengths of the attractive forces between the molecules or other atomic-scale particles that make up the substance, that is, on intermolecular forces (IMFs). As temperature increases, the kinetic energies (KEs) of molecules also increase; this provides the energy required to overcome IMFs and thus increase the distance between particles as a substance changes from solid to liquid to gas. These intermolecular forces (forces between one molecule and another) can vary widely, but they are usually weaker than the chemical bonding forces within each molecule (intramolecular forces). See Figure 1.
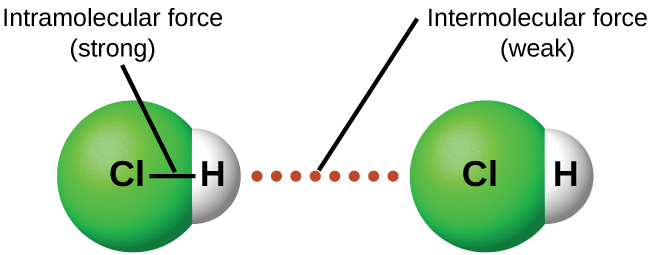
When liquid HCl boils, HCl molecules that were close together move much further apart. Vaporizing liquid HCl at its boiling point requires 16.2 kJ/mol. This is the energy required to overcome the IMFs between HCl molecules. To break the H-Cl covalent bond requires the bond enthalpy, 431 kJ/mol, about 25 times more energy. Thus, when HCl boils the molecules do not break apart into H atoms and Cl atoms. This is true for almost all substances: boiling separates molecules but does not separate the atoms that make up the molecules. Also, note that enthalpy of vaporization and boiling point are indicators of the relative strengths of IMFs.
D13.2 Molecular Polarity
In Section 10.3 we described polar bonds—bonds in which there is separation of charge due to different electronegativities of the bonded atoms. For example, the bond in the HCl molecule is polar because Cl is more electronegative than H. Because of the polar bond, HCl has a molecular dipole moment. Molecules that have a dipole moment are called polar molecules.
A good way to predict whether a molecule is polar is first to determine whether there are polar bonds. If there are no polar bonds, the molecule is nonpolar. Even if there are polar bonds, it is possible for the bond dipoles to cancel. For example, both carbon dioxide and sulfur dioxide have polar bonds, but only sulfur dioxide is polar. The reason is that in carbon dioxide, which is a linear molecule according to VSEPR, the bond dipoles are equal and pointing in exactly opposite directions. This results in a molecular dipole moment of zero. In sulfur dioxide, which is bent, the bond dipoles are at an angle and the resultant molecular dipole is not zero.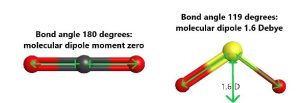
Bond dipoles behave as vectors, so if you are familiar with vector addition you can predict when bond dipoles cancel and when they do not. Here is another way to predict: molecules with all terminal atoms the same and no lone pairs on the central atom are nonpolar because of cancellation of bond dipoles. (In the case of a molecule with an odd number of electrons, a single electron on the central atom is equivalent to a lone pair in this rule.) For molecules with more than a single central atom, predicting dipoles becomes trickier. If atoms have similar electronegativities bond dipoles are weak and therefore molecular dipole moments are small. An example of this last statement is hydrocarbons: because the electronegativity difference between C and H is small bonds have low polarity and hydrocarbons are essentially nonpolar.
Exercise 1: Predicting Molecular Polarity
D13.3 London Forces
All molecules are weakly attracted to other molecules. Even the noble gases can be condensed to liquids, which implies that there must be attractions between noble-gas atoms. These weak attractive forces are called London forces in honor of German-born American physicist Fritz London who, in 1928, first explained them. (London forces are also called dispersion forces or instantaneous dipole–instantaneous dipole forces.)
Consider a hydrogen molecule. Typically the electron density distribution around the two H-atom nuclei would be symmetric. However, for a very brief instant the electron density might become slightly unsymmetric—say more electron density on the left side of the H2 molecule. During that brief instant there is a dipole in the H2 molecule pointing left. That dipole can induce a temporary dipole in another H2 molecule nearby. These two temporary dipoles cause electrostatic attraction between the two neighboring molecules, as illustrated in Figure 2.
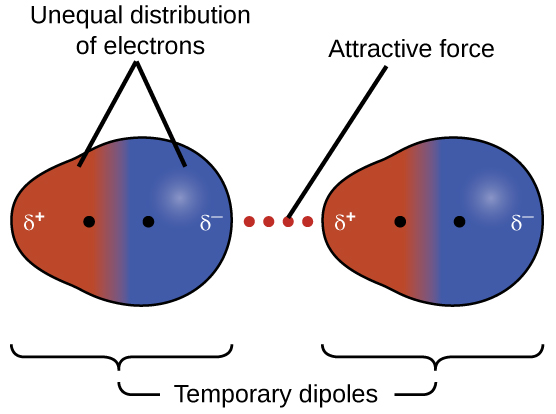
London forces are significant only when the molecules are very close to each other—the force is proportional to [latex]\dfrac{1}{R^6}[/latex] where R is the distance between molecules. London forces are larger the greater the number of electrons in a molecule. In a larger molecule, the valence electrons are, on average, farther from the nuclei than in a smaller molecule. Thus, electrons are held less tightly and can more easily form instantaneous dipoles. For example, consider the halogen diatomic molecules, which have increasing number of electrons as you go down the group. At room temperature, F2 and Cl2 are gases, Br2 is a liquid, and I2 is a solid (see Table 1).
| Halogen | Number of e- | Atomic Radius | Melting Point | Boiling Point |
|---|---|---|---|---|
| fluorine, F2 | 18 | 72 pm | 53 K | 85 K |
| chlorine, Cl2 | 34 | 99 pm | 172 K | 238 K |
| bromine, Br2 | 70 | 114 pm | 266 K | 332 K |
| iodine, I2 | 106 | 133 pm | 387 K | 457 K |
| astatine, At2 | 170 | 150 pm | 575 K | 610 K |
| Table 1. Melting and Boiling Points of the Halogens | ||||
The measure of how easy it is for another electrostatic charge to distort a molecule’s electron distribution is known as polarizability. The more polarizable a molecule is the larger the London forces between molecules are.
Example 1
London Forces and Their Effects
Order these compounds from lowest to highest boiling point: CH4, SiH4, GeH4, and SnH4. Explain your reasoning.
Solution
All of these molecules have a symmetric tetrahedral molecular geometry, and therefore are nonpolar. These compounds experience only London forces. Hence, the molecule with fewer electrons will be less polarizable and have weaker London forces. The number of electrons in CH4, SiH4, GeH4, and SnH4 are 10, 18, 36, and 54, respectively. Therefore, the order from lowest to highest boiling point is expected to be CH4 < SiH4 < GeH4 < SnH4.
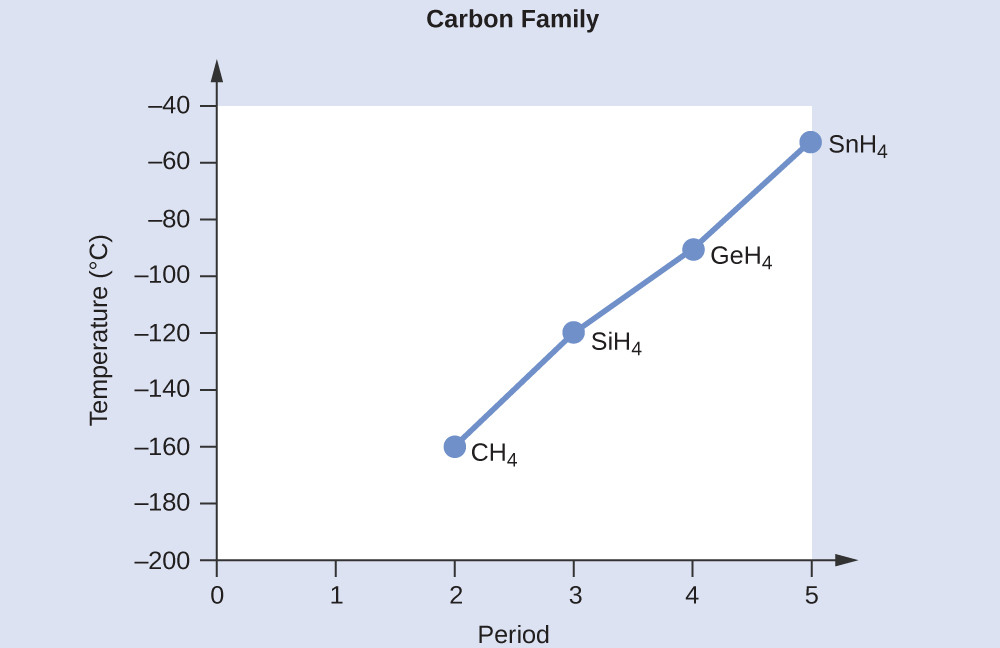
A graph of the actual boiling points of these compounds versus the period of the group 14 element shows this prediction to be correct.
Check Your Learning
Order the following hydrocarbons from lowest to highest boiling point: ethane (C2H6), propane (C3H8), and n-butane (C4H10).
Answer:
C2H6 < C3H8 < C4H10. All of these linear alkanes are essentially nonpolar and only have London forces, therefore the longer the linear alkane chain is the stronger the London forces are and the higher boiling point is.
Molecular geometry also affects the strength of London forces For example, boiling points for n-pentane, isopentane, and neopentane (Figure 3) are 36 °C, 27 °C, and 9.5 °C, respectively, indicating that London forces are greatest for n-pentane and least for neopentane. These three molecules are constitutional isomers and therefore have same number of electrons. In n-pentane, the linear shape provides a greater surface area between molecules when they come in contact, resulting in stronger London forces. Neopentane has the most compact geometry of the three, yielding the smallest surface area for intermolecular contact and, hence, the weakest London forces.
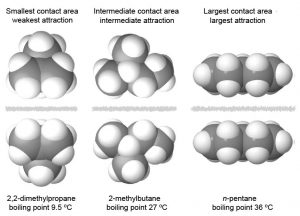
Exercise 2: Predicting Boiling Points
D13.4 Dipole-Dipole Attractions
Polar molecules have a partial positive charge on one side and a partial negative charge on the other side—a permanent separation of charge. Dipole-dipole attractions are attractive forces between molecules when the positive end of one dipole interacts with the negative end of another dipole (Figure 4).
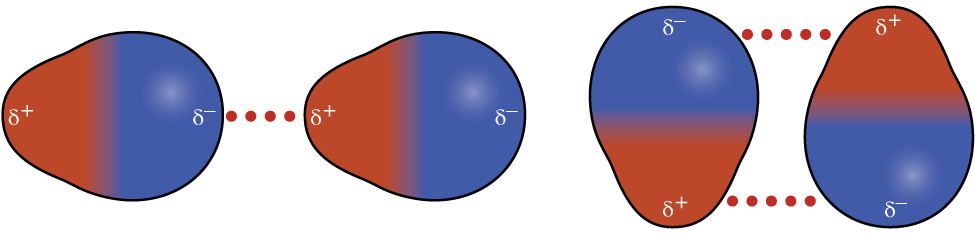
The effect of dipole-dipole interaction is apparent when we compare the polar HCl to the nonpolar F2. Each molecule has 18 electrons and approximately the same shape, and therefore similar London forces. The higher boiling point of HCl (188 K) compared to F2 (85 K) is a reflection of the additional dipole-dipole attractions between HCl molecules.
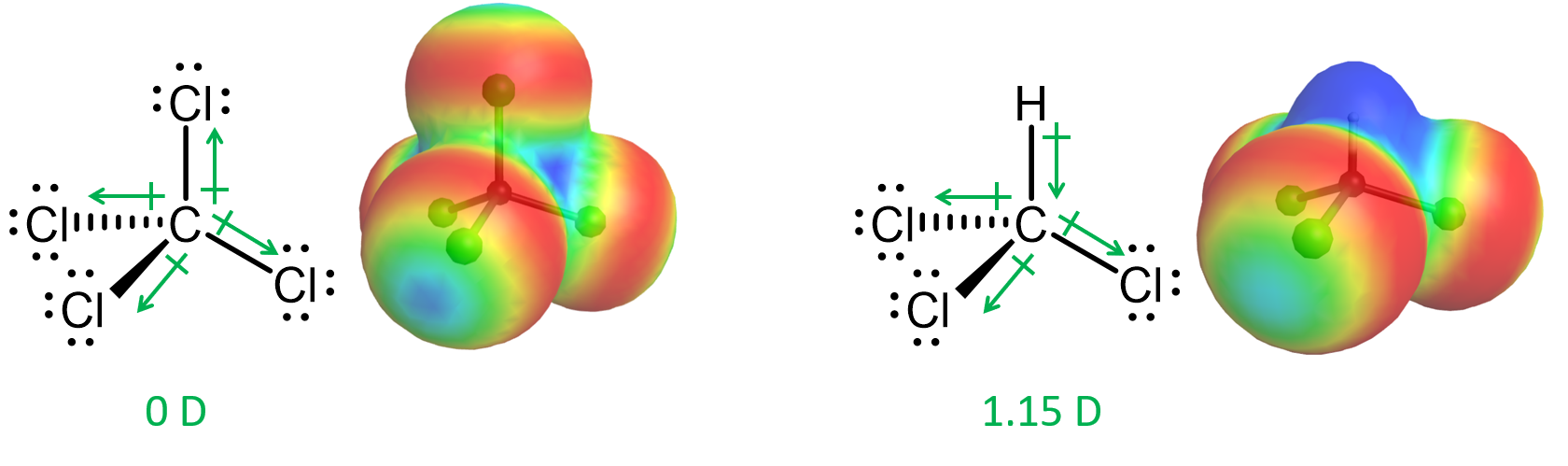
The strength of dipole-dipole interaction is dependent on the molecular dipole moment—the larger the molecular dipole moment, the stronger the dipole-dipole interactions. Remember that the molecular dipole moment depends on geometry as well as polarity of individual bonds. For example, a C-Cl bond is quite polar (about 1.9 D), but tetrachloromethane, CCl4, is a nonpolar molecule because the four polar C-Cl bonds are arranged symmetrically in a tetrahedral geometry, and the bond dipoles cancel (Figure 5). On the other hand, trichloromethane, CHCl3 (chloroform), with three C-Cl bonds and a C-H bond, has a molecular dipole moment of 1.15 D (Figure 5).
Example 2
Dipole-Dipole Forces and Their Effects
Predict which has higher boiling point: N2 or CO. Explain your reasoning.
Solution
CO and N2 are both diatomic molecules with 14 electrons, so they experience similar London forces. Because CO is a polar molecule, it also experiences dipole-dipole attractions. Because N2 is nonpolar, there are no dipole-dipole attractions. The additional dipole-dipole attractions between CO molecules result in a higher boiling point. [The boiling point of N2 is 77 K, the boiling point of CO is 82 K.]
Check Your Learning
Predict which has higher boiling point: ICl or Br2. Explain your reasoning.
Answer:
ICl has higher boiling point. ICl and Br2 have the same number of electrons (70) and therefore experience similar London forces. ICl is polar and thus also exhibits dipole-dipole attractions; Br2 is nonpolar and does not. The additional dipole-dipole attractions require additional energy to overcome, so ICl has a higher boiling point.
D13.5 Hydrogen Bonding
Water (H2O, 10 electrons) has a much higher boiling point (373 K) than nitrosyl fluoride (ONF, 24 electrons, boiling point = 201 K). These two molecules have similar shapes with ONF having more electrons, and they also have comparable dipole moments. The large difference in boiling points cannot be explained by London forces or dipole-dipole interactions. Instead, it is due to an additional IMF, called hydrogen bonding, that occurs in water but not ONF. A hydrogen bond is the attraction of a hydrogen atom in a molecule or part of a molecule X–H for another atom or group of atoms.
Strong hydrogen bonding occurs between a hydrogen atom that is covalently bonded to F, O, or N and an electron lone pair on another F, O, or N atom. F, O, and N, are among the most electronegative elements in the periodic table, a characteristic necessary for strong hydrogen bonding. If we designate a hydrogen bond as X-H···Z, where X-H represents the molecule with the hydrogen atom and Z represents the other atom or group of atoms, then, if X is one of the atoms N, F, or O and Z is also one of the atoms N, F, or O, there is strong hydrogen bonding.
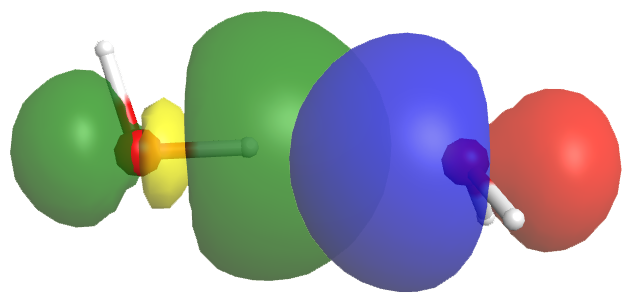
Despite the word “bond,” a hydrogen bond is about 5-10% the strength of a typical covalent bond. Some of hydrogen bonding attractions comes from dipole-dipole attraction, but more important is partial electron sharing that resembles the formation of a covalent bond. Figure 6 shows the hydrogen bond interaction between two water molecules. The orbital of the lone pair (blue/red) on the right-hand water molecule overlaps with an empty O-H antibonding orbital (yellow/green) of the other water molecule. This overlap allows electron density to be shared between the two water molecules. There is less electron density than in a covalent bond, but enough to cause significant attraction.
A water molecule has two lone pairs on O and two O-H bonds. If you consider a sample of pure water, on average, each water molecule can participate in four hydrogen bonds (Figure 7). In contrast, HF has three lone pairs on F and only one F-H bond. In a sample of pure HF each HF molecule can participate in two hydrogen bondson average (there is an excess of lone pairs compared to F-H).
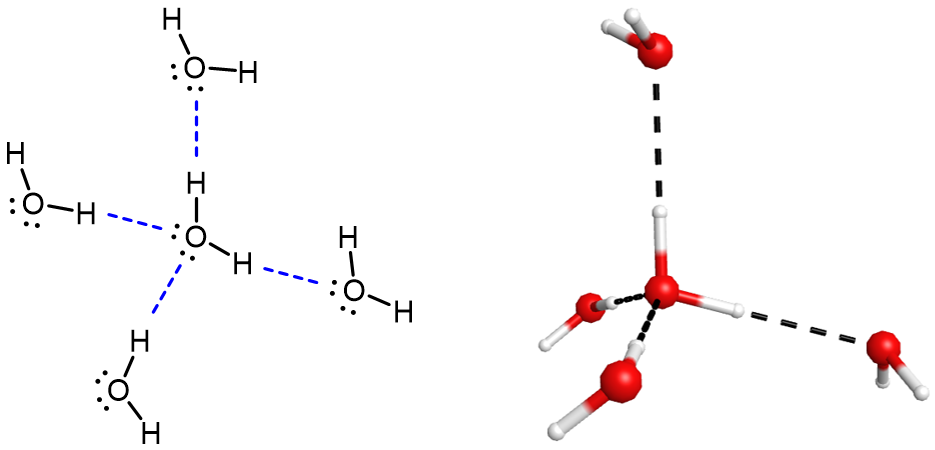
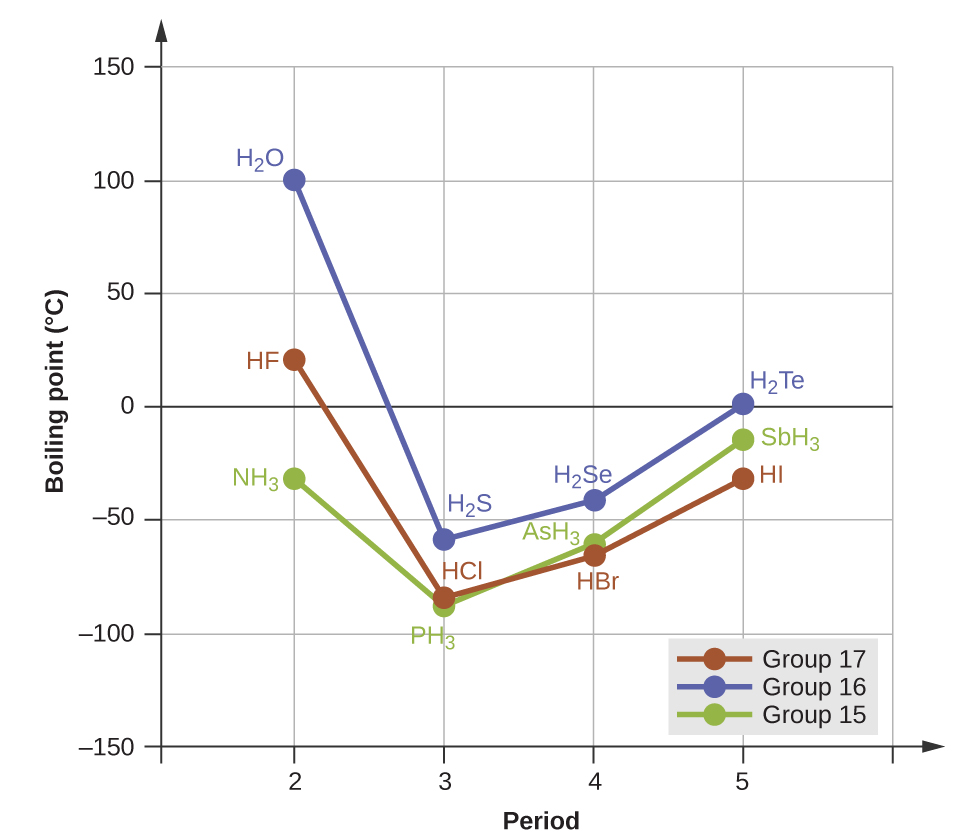
For small molecules, presence of hydrogen bonding has a pronounced effect on a substance's properties. For example, consider the boiling point trends shown in Figure 8. Consider the hydrogen halides (red on the graph). Going down the group, the polarities of the molecules decrease while the number of electrons increases substantially. For Cl, Br, and I, the effect of stronger London forces dominates that of slightly weaker dipole-dipole attractions, and the experimental boiling points increase steadily. For HF, however, the boiling point is dramatically higher than this trend predicts, providing compelling evidence for the presence of strong hydrogen bonding in HF compared to HCl, HBr, or HI. The same trend is observed in the oxygen group and the nitrogen group.
Example 3
Effect of Hydrogen Bonding on Boiling Points
Predict whether the boiling point of methylamine (CH3NH2) is higher, lower, or about the same as the boiling point of ethane (CH3CH3, boiling point −89 °C). Explain your reasoning.
Solution
The boiling point for methylamine should be significantly greater than that of ethane. CH3CH3 and CH3NH2 are similar in molecular geometry and number of electrons, but methylamine, with an −NH2 group, is a polar molecule, has dipole-dipole attractions, and hydrogen bonding. This greatly increases its IMFs, and therefore its boiling point. (The experimental boiling point is −6 °C.)
Exercise 3: Intermolecular Forces and Boiling Points
Consider the compounds dimethyl ether (CH3OCH3), ethanol (CH3CH2OH), and propane (CH3CH2CH3). Their boiling points, not in order, are −42.1 °C, −24.8 °C, and 78.4 °C. Match each compound with its boiling point. Explain your reasoning.
Podia Question
Consider the data in this table. (Note that temperatures are in Kelvins.)
: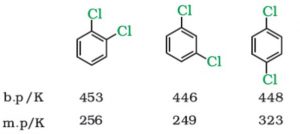
1. Which of the substances are solids and which are liquids at room temperature?
2. Analyze the data for boiling point (b.p.). Write at least two useful ways you might interpret the data. (For example, one useful interpretation could be, "The boiling points are all about the same." You can include this one.)
3. Using ideas developed in this chapter, write an explanation for each of the two interpretations of the data you developed in part 2.
4. Analyze the data for melting point (m.p.). Do the melting-point data follow the same trend as the boiling-point data? What is the biggest difference?
5. (This is a hard one.) Without consulting any source other than your brain, suggest an explanation of the major difference in trends of m.p. and b.p. Think about the positions and motions of atomic-scale particles in solids, liquids, and gases and how those differences might affect transitions from solid to liquid differently from transitions from liquid to gas.
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.

