Lab 9: AT-HOME EXPERIMENT
Expected time requirement: About 60 minutes.
Protein Folding and Denaturation
Proteins are composed of secondary structures that primarily result from non-covalent, intramolecular interactions between the backbone amides. Additionally, entropy favors the reduction of solvent-accessible surface area of hydrophobic amino acid residues. This promotes hydrophobic residues being pushed into the center of a protein, thus organizing the hydrophobic core that stabilizes globular protein structures.
Some chemical agents can compete for those weak intramolecular interactions and reduce the entropic penalty of organizing water near hydrophobic residues. This disrupts protein structure, leading to denaturation. Heat can also disrupt protein structure, by increasing kinetic energy and causing the molecules to vibrate so rapidly that the weak interactions are broken.
Protein denaturation is an essential part of food science, and an everyday part of cooking. In this experiment you will get to explore the phenomenon yourself using eggs. Egg whites contain ~90% water and ~10% protein. The most abundant protein in egg whites is ovalbumen, shown below. It is a 386 amino acid protein with a fairly compact structure containing both beta sheets and alpha helices. You will study the denaturation of ovalbumin in the experiment below.
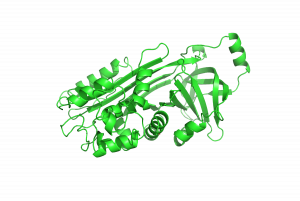
Denaturation of Albumin
Materials
- Stove and pot OR microwave and microwave-safe bowl
- 1 fork
- 1 pair scissors
- 1-4 eggs
- 1 bowl
- 4 small containers of about the same size
- 2/3 cup water
- 1/3 cup rubbing alcohol
Procedure
- Pour 1/3 cup rubbing alcohol into one small container, room temperature water (1/3 cup) into another, and the rest of the water (1/3 cup) into a microwave-safe container (or into a pot).
- Crack egg into the bowl, removing the yolks. (This is not super easy if you’ve never done it before. Dr. Prost’s preferred approach is to use one half of the broken egg shell as a little cup for the yolk while letting the whites run out into the bowl. You can search YouTube for “how to separate egg whites and egg yolks” for more help.)
- If using 1 or 2 eggs, cut the egg white into pieces using scissors so you can more easily add ¼ of the total into each glass container. You can alternatively just use 4 eggs.
- Heat up the water for your hot water treatment and pour into one of the empty glass containers.
- Quickly put ¼ of the egg whites into the boiling water. Then put ¼ into the alcohol, ¼ into the room temperature water, and the remainder into the final, empty glass container.
- Observe any immediate changes that occur in terms of egg white color and consistency. If you try stirring the different treatments, rinse your fork between stirs.
- Wait for 30 minutes. While you’re waiting, check out the following protein folding simulations:
- Simulation 1: observe where polar and hydrophobic amino acids reside in different proteins (including albumin!)
- Simulation 2: observe how protein folding is affected by the hydrophobicity/hydrophilicity of its amino acids
- Use the fork to inspect the state of the egg whites in each treatment and note how they may have changed over time.
- Take a photo of your completed experiment (you will have to submit it on the prelab quiz).
Conclusions
So, what happened to the ovalbumin? You will need to answer the following questions on the prelab quiz, and you will discuss them with your partner in lab.
- For each condition tested, answer the following:
- Did you observe protein denaturation?
- If yes, what caused the denaturation at the molecular level?
- If not, why not?
- Describe another method for denaturing proteins in egg whites. What causes the denaturation in this case?
- Can you re-fold proteins that have been denatured? Watch the video below called “How to unboil an egg” and summarize what you learn.

