M17Q7: Kinetics, Equilibrium, and Stability
Learning Objectives
- Distinguish between thermodynamic stability and kinetic stability, and describe the effect of each on whether a reaction is useful in generating products.
A chemical reaction has both kinetic and thermodynamic aspects. The quantity related to kinetics is the rate constant k, which is associated with the activation energy required for the reaction to proceed. Hence, kinetics is related to the reactivity of the reactants. The thermodynamic quantity is the energy difference resulting from the free energy given off during a chemical reaction—the stability of the products relative to the reactants. Although kinetics describes the rates of reactions and how fast equilibrium is reached, it gives no information about conditions once the reaction equilibrates. In the same measure, thermodynamics only gives information regarding the equilibrium conditions of products after the reaction takes place, but does not explain the rate of reaction.
When discussing the concept of stability, it is necessary to distinguish between thermodynamic and kinetic stability.
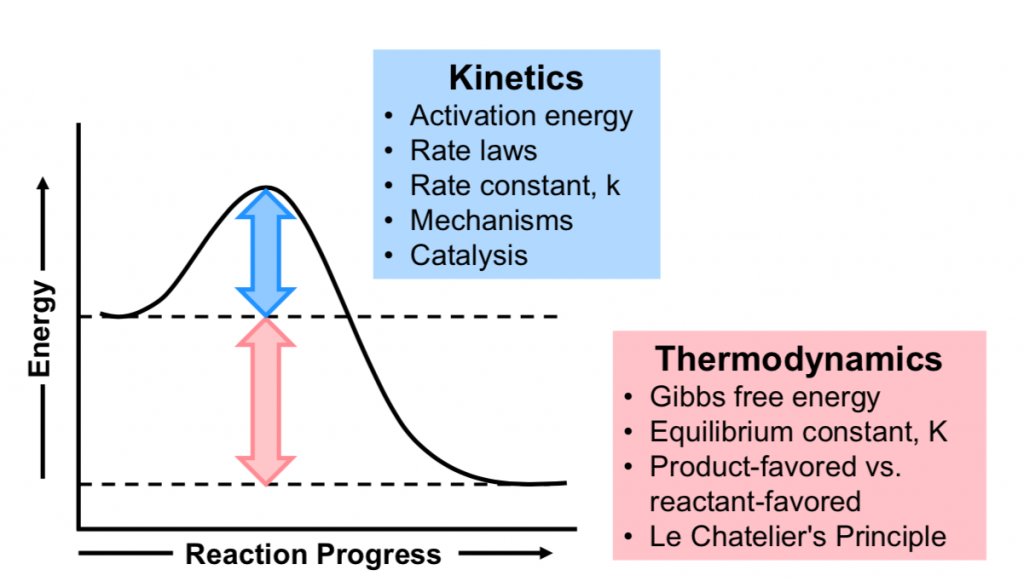
Consider Figure 1. Here the products are at lower energy than the reactants so that ΔG° of the forward reaction is negative. The reaction favors the products and the products are the more thermodynamically stable species.
However, if the reaction barrier (Ea) is high, the reaction may proceed very slowly, and the reactants would be described as being inert (unreactive), i.e., kinetically stable.
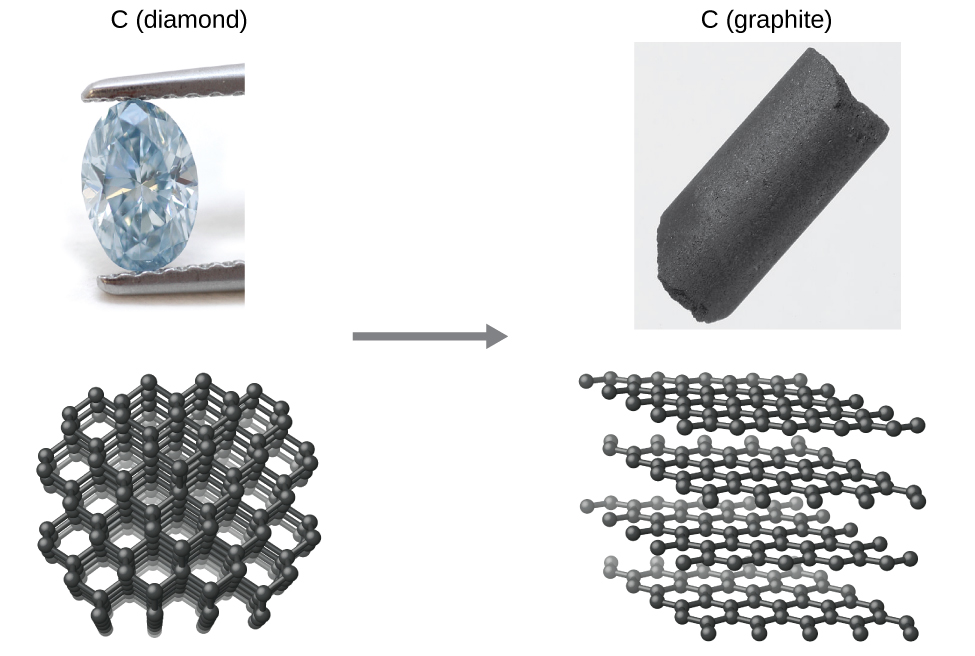
As an example, consider the conversion of diamond into graphite (Figure 2).
C(s, diamond) → C(s, graphite) ΔG° < 0
While diamond is the hardest known material, graphite is one of the softest. This is all due to differences in the way the atoms are bonded together. In diamond, each sp3 carbon is bonded to 4 other sp3 carbon atoms. In graphite each sp2 carbon is bonded to 3 other sp2 carbon atoms in sheets of connected benzene rings. Because the sheets can slide over one another, graphite is slippery.
The relationship between diamond and graphite is a thermodynamic and kinetic one. At normal temperatures and pressures, graphite is more stable than diamond, and the fact that diamond exists at all is due to the very large activation barrier for conversion between the two. There is no easy mechanism for this conversion and so transforming diamond into graphite, or vice versa, requires almost as much energy as destroying the entire lattice and rebuilding it. Once diamond is formed, therefore, it cannot reconvert back to graphite because the barrier is too high. So diamond is kinetically stable (due to the high activation energy barrier), but not thermodynamically stable (due to ΔG°rxn < 0). Diamond is created deep underground under conditions of extreme pressure and temperature. Under these conditions diamond is actually the more stable of the two forms of carbon, and so over a period of millions of years carbonaceous deposits slowly crystallise into single crystal diamond gemstones.
Key Concepts and Summary
After learning about the effect of enthalpy and entropy on the Gibbs Free Energy and the spontaneity of the reaction, we take a step back to look more holistically at both how fast a reaction proceeds and how many products it creates. The thermodynamic stability is related to the Gibbs Free Energy, dependent only on the energy difference between the reactants and the products. If the Gibbs Free Energy is negative and the reaction is product-favored, the reaction is thermodynamically unstable. If the Gibbs Free Energy is positive and the reaction is reactant-favored, the reaction is thermodynamically stable. The kinetics of the reaction can give information about how fast the reaction will occur. If the activation energy is low, the reaction is kinetically unstable. If the activation energy is high, the reaction is kinetically stable. When discussing if a reaction will occur, it is important to take into account both the kinetic and thermodynamic properties, since a reaction will only occur if both kinetics and thermodynamics are unstable.
Chemistry End of Section Exercises
- Methane is a highly flammable gas. Calculate the ΔG° at 298 K for the combustion of methane. How is possible for methane to exist in our atmosphere? Thermochemical data can be found in Appendix F.
- Combustion of gasoline is an exothermic and entropically-favorable process. How is it possible to store gasoline in a fuel tank without explosions?
- The reaction rates of many spontaneous reactions are actually very slow. Which of these statements is the best explanation for this observation?
- Keq for the reaction is less than one.
- The activation energy of the reaction is large.
- ΔG° for the reaction is positive.
- Such reactions are endothermic.
- The entropy change is negative.
- With thermodynamics, one cannot determine ____. (Select all that apply)
- the speed of a reaction
- the direction of a spontaneous reaction
- the extent of a reaction
- the temperature at which a reaction will be spontaneous
Answers to Chemistry End of Section Exercises
- Methane combusts according to the reaction: CH4(g) + 2 O2(g) ⇌ CO2(g) + 2 H2O(g). ΔG° = -800.8 kJ/mol. Methane is a thermodynamically unstable compound, however, since the compound does exist in our atmosphere, it must be a kinetically stable compound. The reaction to combust methane is slow, unless initial energy is put into the system to overcome the activation energy of the reaction.
- The gas in a fuel tank does not explode while stored in a tank. Fuel is unreactive under standard conditions; it takes a spark to provide the activation energy to the reactants, beginning the process of combustion.
- B
- A
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

