M2Q1: Protons, Neutrons, and Electrons
Introduction
This section explores atoms. Atoms are the building blocks of matter, but what are the building blocks of the atom? Here, we will introduce fundamental aspects of modern atomic theory. We’ll examine three types of subatomic particles: protons, neutrons, and electrons. In addition, we’ll interpret atomic symbols. The section below provides a more detailed description of these topics, worked examples, practice problems and a glossary of important terms.
Learning Objectives for Subatomic Particles
- Recognize the atom is comprised of protons, neutrons, and electrons; distinguish among these building blocks.
| The Structure of the Atom | - Know the names and symbols of certain elements.
| Chemical Symbols |
| Key Concepts and Summary | Glossary | End of Section Exercises |
The Structure of the Atom
Much of our current understanding of atoms and atomic structure was developed in the early part of the 20th century. The work of Thomson, Millikan, and others revealed the charge and mass of the negative subatomic particles—the electrons. We designate electrons with the symbol “e–“, the superscript negative sign emphasizes their negative charge. The surprising results of Rutherford’s experiments, which we will explore in more detail in the next section, indicated that the positively charged subatomic particles—the protons (p+)—are much more massive than electrons (by a factor of about 2,000) and are highly concentrated in the atom’s nucleus, which occupies just a tiny portion of the atom’s entire volume. A third subatomic component that bears no electrical charge—the neutrons (n0)—have masses similar to protons and co-locate with protons in the nucleus.
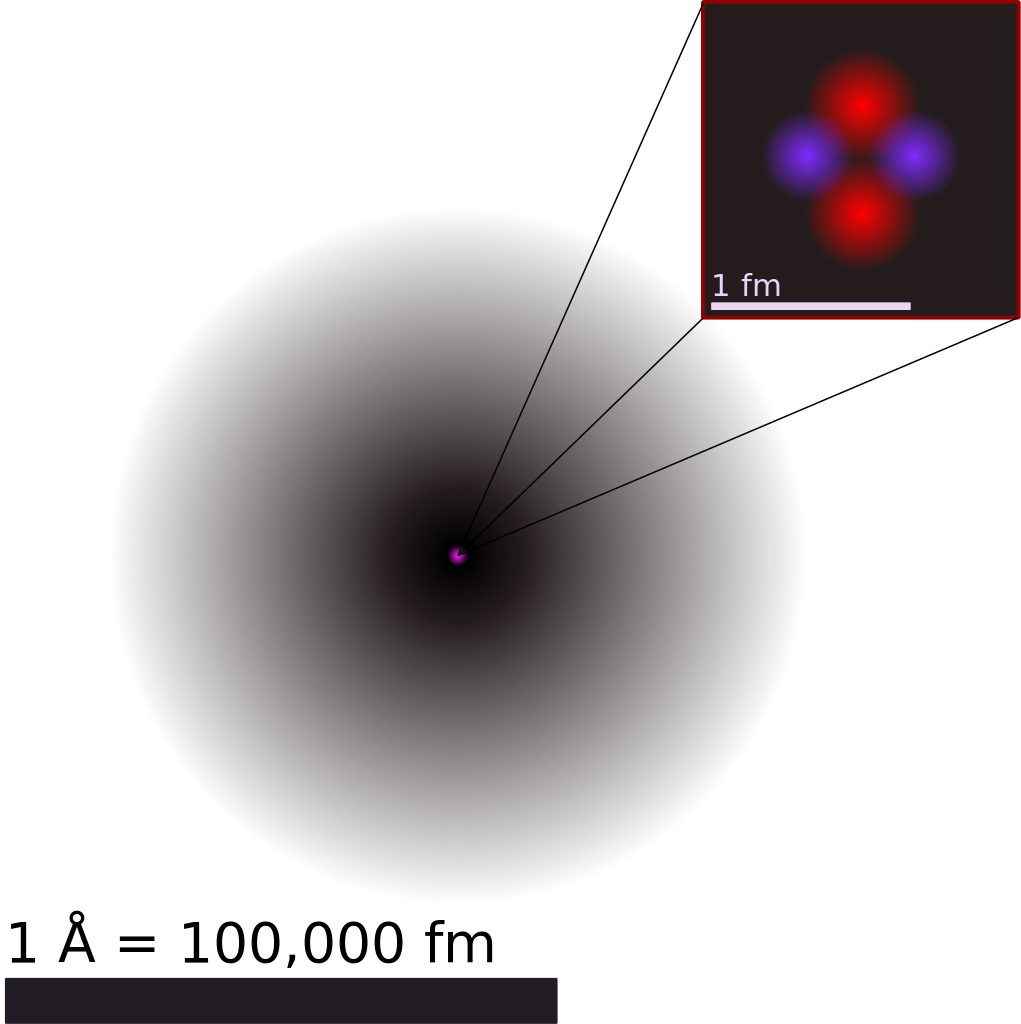
For a perspective about their relative sizes, consider this: if the nucleus were the size of a blueberry, the atom would be about the size of a football stadium (Figure 1).

Most of the volume of an atom is occupied by the “cloud” of electrons. We use the term “electron cloud” because the exact positions of the electrons cannot be known, we simply know that they are distributed about the volume of the atom (Figure 2). More details about the energies and distributions of electrons in the atom will be discussed in a later module on light, matter, and atomic structure.
Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2 × 10−23 g, and an electron has a charge of less than 2 × 10−19 C (coulomb). When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) and the fundamental unit of charge (e). The amu was originally defined based on hydrogen, the lightest element, then later in terms of oxygen. Since 1961, it has been defined with regard to the most abundant isotope of carbon, atoms of which are assigned masses of exactly 12 amu. (This isotope is known as “carbon-12” as will be discussed later in a discussion of isotopes.) Thus, one amu is exactly ![]() of the mass of one carbon-12 atom. Moreover, 1 amu = 1.6605 × 10−24 g. (The Dalton (Da) and the unified atomic mass unit (u) are alternative units that are equivalent to the amu.) The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10−19 C.
of the mass of one carbon-12 atom. Moreover, 1 amu = 1.6605 × 10−24 g. (The Dalton (Da) and the unified atomic mass unit (u) are alternative units that are equivalent to the amu.) The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10−19 C.
A proton has a mass of 1.0073 amu and a charge of 1+. A neutron is a slightly heavier particle with a mass 1.0087 amu and a charge of zero; as its name suggests, it is neutral. The electron has a charge of 1− and is a much lighter particle with a mass of 0.0005486 amu (it would take 1836 electrons to equal the mass of one proton). The properties of these fundamental particles are summarized in Table 1. (An observant student might notice that the sum of an atom’s subatomic particles does not equal the atom’s actual mass: The total mass of six protons, six neutrons, and six electrons is 12.099 amu, slightly larger than 12.00 amu. This “missing” mass is known as the mass defect, which is beyond the scope of this course.)
| Name | Location | Charge (C) | Unit Charge | Mass (amu) | Mass (g) |
| electron | outside nucleus | −1.602 × 10−19 | 1− | 0.0005486 | 0.0009109 × 10−28 |
| proton | nucleus | 1.602 × 10−19 | 1+ | 1.00728 | 1.67262 × 10−24 |
| neutron | nucleus | 0 | 0 | 1.00866 | 1.67493 × 10−24 |
Example 1
Mass Interconversion
If a person’s mass is 100 kg, what is their mass in amu’s?
Solution
1 amu = 1.6605 × 10−24 g.
Therefore the person’s mass is given by:
100 kg × ![]() ×
× ![]() = 6.022 × 1028 amu
= 6.022 × 1028 amu
Check Your Learning
What is the mass in grams of a proton that has mass of 1.0073 amu?
Answer:
1.6726 × 10-24 g
The number of protons in the nucleus of an atom is its atomic number (Z). This is the defining trait of an element: its value determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons in a neutral atom. Therefore, the atomic number also indicates the number of electrons in an atom. The total number of protons and neutrons in an atom is called its mass number (A). The number of neutrons is therefore the difference between the mass number and the atomic number: A – Z = number of neutrons.
atomic number (Z) = number of protons
mass number (A) = number of protons + number of neutrons
A – Z = number of neutrons
Isotopes of an element are atoms with the same atomic number but different mass numbers; isotopes of an element, therefore, differ from each other only in the number of neutrons within the nucleus. We will discuss isotopes in greater detail later.
Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. When the numbers of these subatomic particles are not equal, the atom is electrically charged and is called an ion. The charge of an atom is defined as:
Atomic charge = number of protons − number of electrons
As will be discussed in more detail later when we talk about monoatomic ions, atoms (and molecules) typically acquire charge by gaining or losing electrons. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Positively charged atoms called cations are formed when an atom loses one or more electrons. For example, a neutral sodium atom (Z = 11) has 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+). A neutral oxygen atom (Z = 8) has eight electrons, and if it gains two electrons it will become an anion with a 2- charge (8 − 10 = 2-).
Example 2
Composition of an Atom
Iodine is an essential trace element in our diet; it is needed to produce thyroid hormone. Insufficient iodine in the diet can lead to the development of a goiter, an enlargement of the thyroid gland (Figure 3).
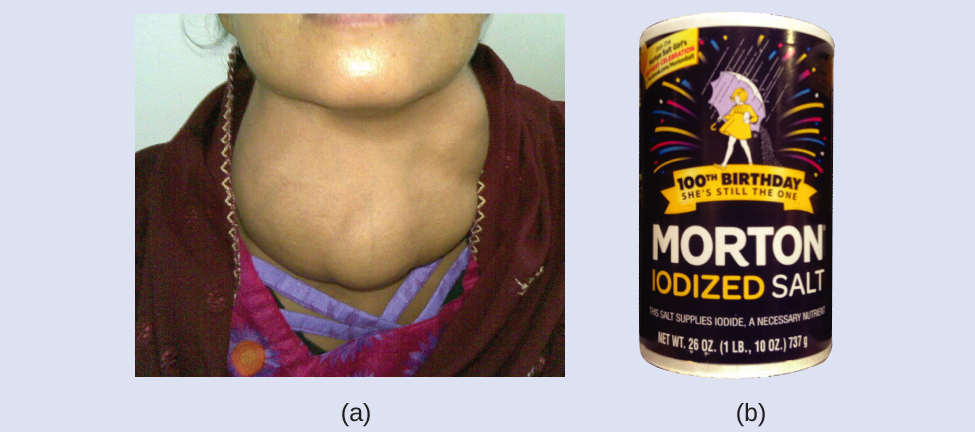
The addition of small amounts of iodine to table salt (iodized salt) has essentially eliminated this health concern in the United States, but as much as 40% of the world’s population is still at risk of iodine deficiency. The iodine atoms are added as anions, and each has a 1− charge and a mass number of 127. Determine the numbers of protons, neutrons, and electrons in one of these iodine anions.
Solution
The atomic number of iodine (53) tells us that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus. Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 – 53 = 74). Since the iodine is added as a 1- anion, the number of electrons is 54 [53 – (1–) = 54].
Check Your Learning
An ion of platinum has a mass number of 195 and contains 74 electrons. How many protons and neutrons does it contain, and what is its charge?
Answer:
78 protons; 117 neutrons; charge is 4+
Chemical Symbols
A chemical symbol is an abbreviation that we use to indicate an element or an atom of an element. For example, the symbol for mercury is Hg (Figure 4). We use the same symbol to indicate one atom of mercury (microscopic domain) or to label a container of many atoms of the element mercury (macroscopic domain).

No doubt you are familiar with many elements and their symbols already, but the ones that you should know for this course are listed in Table 2. These are the names and symbols for elements 1 through 36 as well as some other commonly encountered elements. Some symbols are derived from the common name of the element; others are abbreviations of the name in another language. Most symbols have one or two letters, but three-letter symbols have been used to describe some elements that have atomic numbers greater than 112. To avoid confusion with other notations, only the first letter of a symbol is capitalized. For example, Co is the symbol for the element cobalt, but CO is the notation for the compound carbon monoxide, which contains atoms of the elements carbon (C) and oxygen (O). All known elements and their symbols are in the periodic table in Appendix A.
| Element | Symbol | Element | Symbol | Element | Symbol |
| hydrogen | H | sulfur | S | gallium | Ga |
| helium | He | chlorine | Cl | germanium | Ge |
| lithium | Li | argon | Ar | arsenic | As |
| beryllium | Be | potassium | K | selenium | Se |
| boron | B | calcium | Ca | bromine | Br |
| carbon | C | scandium | Sc | krypton | Kr |
| nitrogen | N | titanium | Ti | ||
| oxygen | O | vanadium | V | ||
| fluorine | F | chromium | Cr | silver | Ag |
| neon | Ne | manganese | Mn | gold | Au |
| sodium | Na | iron | Fe | barium | Ba |
| magnesium | Mg | cobalt | Co | iodine | I |
| aluminum | Al | nickel | Ni | mercury | Hg |
| silicon | Si | copper | Cu | lead | Pb |
| phosphorus | P | zinc | Zn | uranium | U |
Traditionally, the discoverer (or discoverers) of a new element names the element. However, until the name is recognized by the International Union of Pure and Applied Chemistry (IUPAC), the recommended name of the new element is based on the Latin word(s) for its atomic number. For example, element 106 was called unnilhexium (Unh), element 107 was called unnilseptium (Uns), and element 108 was called unniloctium (Uno) for several years. These elements are now named after scientists (or occasionally locations); for example, element 106 is now known as seaborgium (Sg) in honor of Glenn Seaborg, a Nobel Prize winner who was active in the discovery of several heavy elements.

Visit this site to learn more about IUPAC, the International Union of Pure and Applied Chemistry, and explore its periodic table.
Key Concepts and Summary
An atom consists of a small, positively charged nucleus surrounded by electrons. The nucleus contains protons and neutrons; its diameter is about 20,000-60,000 times smaller than that of the atom. The mass of one atom is usually expressed in atomic mass units (amu), which is defined as exactly ![]() of the mass of a carbon-12 atom and is equal to 1.6605 × 10−24 g.
of the mass of a carbon-12 atom and is equal to 1.6605 × 10−24 g.
Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons are relatively heavy particles with no charge and a mass of 1.0087 amu. Electrons are light particles with a charge of 1- and a mass of 0.0005486 amu. The number of protons in the nucleus is called the atomic number (Z) and is the property that defines an atom’s elemental identity. The sum of the numbers of protons and neutrons in the nucleus is called the mass number and, is approximately equal to the mass of the atom expressed in amu. An atom is neutral when it contains equal numbers of electrons and protons.
A chemical symbol identifies the atoms in a substance using symbols, which are one-, two-, or three-letter abbreviations for the atoms.
Glossary
- anion
- negatively charged atom or molecule (contains more electrons than protons)
- atomic mass
- average mass of atoms of an element, expressed in amu
- atomic mass unit (amu)
- (also, unified atomic mass unit, u, or Dalton, Da) unit of mass equal to
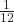 of the mass of a 12C atom
of the mass of a 12C atom
- atomic number (Z)
- number of protons in the nucleus of an atom
- cation
- positively charged atom or molecule (contains fewer electrons than protons)
- chemical symbol
- one-, two-, or three-letter abbreviation used to represent an element or its atoms
- Dalton (Da)
- alternative unit equivalent to the atomic mass unit
- electron
- negatively charged, subatomic particle of relatively low mass located outside the nucleus
- fundamental unit of charge
- (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10−19 C
- ion
- electrically charged atom or molecule (contains unequal numbers of protons and electrons)
- mass number (A)
- sum of the numbers of neutrons and protons in the nucleus of an atom
- neutron
- uncharged, subatomic particle located in the nucleus
- nucleus
- massive, positively charged center of an atom made of protons and neutrons
- proton
- positively charged, subatomic particle located in the nucleus
- unified atomic mass unit (u)
- alternative unit equivalent to the atomic mass unit
Chemistry End of Section Exercises
- In what way are isotopes of a given element always different? In what way(s) are they always the same?
- Identify characteristics of the following subatomic particles by putting an “X” in the appropriate box if the description applies to that particle.
Protons Neutrons Electrons A major contributor to an atom’s mass A major contributor to an atom’s volume A charged particle Two neutral atoms of the same element may have different numbers of these present - Write the symbol for each of the following ions:
- the ion with a 1+ charge, atomic number 55, and mass number 133
- the ion with 54 electrons, 53 protons, and 74 neutrons
- the ion with atomic number 15, mass number 31, and a 3- charge
- the ion with 24 electrons, 30 neutrons, and a 3+ charge
- Open the Build an Atom simulation and click on the Atom icon.
- Pick any one of the first 10 elements that you would like to build and state its symbol.
- Drag protons, neutrons, and electrons onto the atom template to make an atom of your element. State the numbers of protons, neutrons, and electrons in your atom, as well as the net charge and mass number.
- Click on “Net Charge” and “Mass Number,” check your answers to (b), and correct, if needed.
- Predict whether your atom will be stable or unstable. State your reasoning.
- Check the “Stable/Unstable” box. Was your answer to (d) correct? If not, first predict what you can do to make a stable atom of your element, and then do it and see if it works. Explain your reasoning.
- Open the Build an Atom simulation
- Drag protons, neutrons, and electrons onto the atom template to make a neutral atom of Lithium-6 and give the isotope symbol for this atom.
- Now remove one electron to make an ion and give the symbol for the ion you have created.
- Determine the number of protons, neutrons, and electrons in the following isotopes that are used in medical diagnoses:
- atomic number 9, mass number 18, charge of 1−
- atomic number 43, mass number 99, charge of 7+
- atomic number 53, atomic mass number 131, charge of 1−
- atomic number 81, atomic mass number 201, charge of 1+
- Name the elements in parts (a), (b), (c), and (d).
- The following are properties of isotopes of two elements that are essential in our diet. Determine the number of protons, neutrons and electrons in each and name them.
- atomic number 26, mass number 58, charge of 2+
- atomic number 53, mass number 127, charge of 1−
- What is the name of the particle that has 17 protons, 18 electrons, and a mass of 37 amu?
Answers to Chemistry End of Section Exercises
- Isotopes always differ in their mass number due to varying numbers of neutrons. Isotopes always have the same number of protons.
-
Protons Neutrons Electrons A major contributor to an atom’s mass X X A major contributor to an atom’s volume X A charged particle X X Two neutral atoms of the same element may have different numbers of these present X - (a) 133Cs+; (b) 127I–; (c) 31P3−; (d) 57Co3+
Left-click here to watch Exercise 3 problem solving video.
- (a) Carbon-12, 12C;
(b) This atom contains six protons and six neutrons. There are six electrons in a neutral 12C atom. The net charge of such a neutral atom is zero, and the mass number is 12.
(c) The preceding answers are correct.
(d) The atom will be stable since C-12 is a stable isotope of carbon.
(e) The preceding answer is correct. Other answers for this exercise are possible if a different element of isotope is chosen. - (a) Lithium-6 contains three protons, three neutrons, and three electrons. The isotope symbol is 6Li or
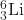 .
.
(b) 6Li+ or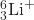
- (a) 9 protons, 9 neutrons, 10 electrons;
(b) 43 protons, 56 neutrons, 36 electrons;
(c) 53 protons, 78 neutrons, 54 electrons;
(d) 81 protons, 120 neutrons, 80 electrons;
(e) a) Flourine-18; b) Technetium-99; c) Iodine-131; d) Thallium-201 - (a) Iron-58, 26 protons, 24 electrons, and 32 neutrons;
(b) Iodine-127, 53 protons, 54 electrons, and 74 neutrons - Chloride ion (Cl–)
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

