M13Q9: Reaction Mechanisms; Elementary Steps
Learning Objectives
- Identify characteristics and components of mechanisms, such as elementary steps and their rate laws, the rate-determining step, and intermediates.
| Key Concepts and Summary | Key Equations | Glossary | End of Section Exercises |
Reaction Mechanisms
A balanced equation for a chemical reaction indicates what is reacting and what is produced, but it reveals nothing about how the reaction actually takes place. The reaction mechanism (or reaction path) is the process, or pathway, by which a reaction occurs.
A chemical reaction usually occurs in steps, although it may not always be obvious to an observer. The decomposition of ozone, for example, appears to follow a mechanism with two steps:
O3(g) → O2(g) + O
O + O3(g) → 2 O2(g)
We call each step in a reaction mechanism an elementary reaction. Elementary reactions occur exactly as they are written and cannot be broken down into simpler steps. Elementary reactions must add up to the overall reaction, which, for the decomposition of ozone, is:
2 O3(g) → 3 O2(g)
Notice that the oxygen atom produced in the first step of this mechanism is consumed in the second step and therefore does not appear as a product in the overall reaction. Species that are produced in one step and consumed in a subsequent step are called intermediates.
While the overall reaction equation for the decomposition of ozone indicates that two molecules of ozone react to give three molecules of oxygen, the mechanism of the reaction does not involve the collision and reaction of two ozone molecules. Rather, it involves a molecule of ozone decomposing to an oxygen molecule and an intermediate oxygen atom; the oxygen atom then reacts with a second ozone molecule to give two oxygen molecules.
Because they represent what is actually happening in the reaction on a molecular level, the rate law for an elementary reaction CAN be determined using the stoichiometry of that elementary step. Note that the rate law for an overall reaction that is the sum of more than one elementary reaction CANNOT be determined by simply applying stoichiometry as reactant orders.
| Reaction | Is this an elementary reaction? | Rate Law |
| O3(g) → O2(g) + O | Yes | rate = k[O3] |
| O + O3(g) → 2 O2(g) | Yes | rate = k[O][O3] |
| 2 O3(g) → 3 O2(g) | No – this overall reaction is the result of more than one elementary step | Cannot be determined directly from stoichiometry |
Unimolecular Elementary Reactions
The molecularity of an elementary reaction is the number of reactant species (atoms, molecules, or ions). For example, a unimolecular reaction involves the rearrangement of a single reactant species to produce one or more molecules of product:
A → products
The rate equation for a unimolecular reaction is:
rate = k[A]
In the previous example of the decomposition of ozone,
O3(g) → O2(g) + O
illustrates a unimolecular elementary reaction that occurs as one part of a two-step reaction mechanism. However, some unimolecular reactions may have only a single reaction in the reaction mechanism. (In other words, an elementary reaction can also be an overall reaction in some cases.) For example, the gas-phase decomposition of cyclobutane, C4H8, to ethylene, C2H4, occurs via a unimolecular, single-step mechanism:
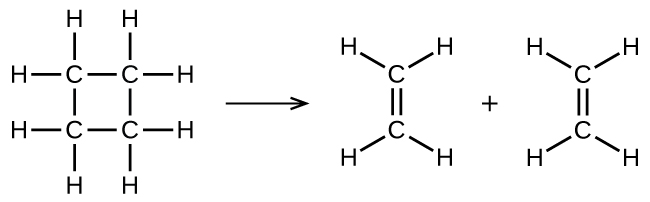
For these unimolecular reactions to occur, all that is required is the separation of parts of single reactant molecules into products.
Chemical bonds do not simply fall apart during chemical reactions. Energy is required to break chemical bonds. The activation energy for the decomposition of C4H8, for example, is 261 kJ per mole. This means that it requires 261 kJ to distort one mole of these molecules into activated complexes that decompose into products:
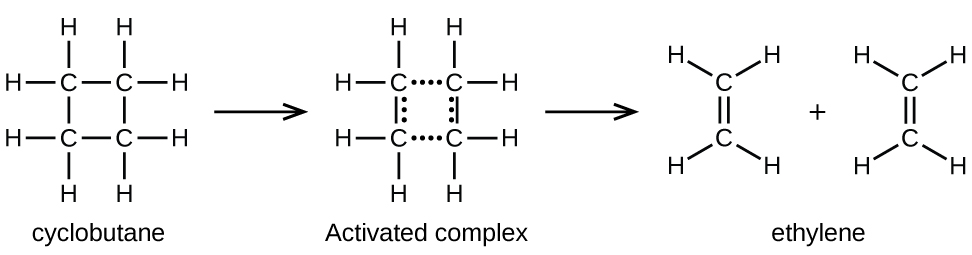
In a sample of C4H8, a few of the rapidly moving C4H8 molecules collide with other rapidly moving molecules and pick up additional energy. When the C4H8 molecules gain enough energy, they can transform into an activated complex, and the formation of ethylene molecules can occur. In effect, a particularly energetic collision knocks a C4H8 molecule into the geometry of the activated complex. However, only a small fraction of gas molecules travel at sufficiently high speeds with large enough kinetic energies to accomplish this. Hence, at any given moment, only a few molecules pick up enough energy from collisions to react.
Bimolecular Elementary Reactions
The collision and combination of two molecules or atoms to form an activated complex in an elementary reaction is called a bimolecular reaction. There are two types of bimolecular elementary reactions:
A + B → products
or
2 A → products
For the first type, in which the two reactant molecules are different, the rate law is first-order in A and first order in B:
rate = k[A][B]
For the second type, in which two identical molecules collide and react, the rate law is second order in A:
rate = k[A][A] = k[A]2
An example of a bimolecular elementary reaction is the decomposition of two hydrogen iodide molecules to produce hydrogen, H2, and iodine, I2, shown in Figure 1:
2 HI(g) → H2(g) + I2(g)
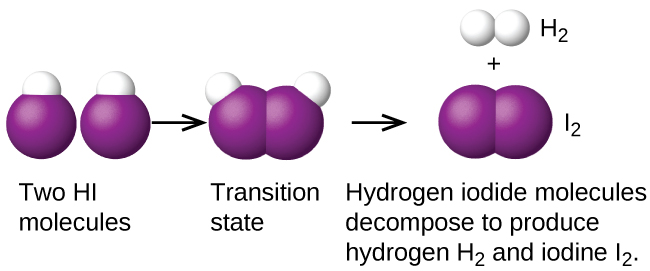
Bimolecular elementary reactions may also be involved as steps in a multistep reaction mechanism. The reaction of atomic oxygen with ozone in the decomposition of ozone is one example:
O + O3(g) → 2 O2(g)
Termolecular Elementary Reactions
An elementary termolecular reaction involves the simultaneous collision of three atoms, molecules, or ions. Termolecular elementary reactions are uncommon because the probability of three particles colliding simultaneously is less than one one-thousandth of the probability of two particles colliding. There are, however, a few established termolecular elementary reactions. The reaction of nitric oxide with oxygen appears to involve termolecular steps:
2 NO(g) + O2(g) → 2 NO2(g)
The rate law is:
rate = k[NO][NO][O2] = k[NO]2[O2]
Key Concepts and Summary
A reaction mechanism gives us insight into how a reaction occurs at the molecular level. Many reactions are not as straightforward as they might appear in a balanced reaction, so we use elementary steps to break down an overall reaction into individual steps. Some elementary reactions occur with only one reactant (unimolecular), while others occur with two reactants (bimolecular, where the reactants can be the same or different). Termolecular reactions occur with three reactants colliding at exactly the same time, which is statistically very rare. Within a reaction mechanism, intermediates often form, which are formed in an early step and then later consumed in a later step. (Side note: catalysts are also involved in the reaction mechanism, but are first consumed as a reactant and then later reformed as a product. They also cancel out when determining the overall reaction).
Key Equations
- unimolecular: A → products
- bimolecular: A + B → products, 2A → products
Glossary
- bimolecular reaction
- the collision and combination of two molecules or atoms to produce one or more products or activated complexes in an elementary reaction
- elementary reaction
- a single step in a reaction mechanism that occurs exactly as it is written and cannot be broken down into simpler steps
- intermediate
- a reaction species that is produced in one step and consumed in a subsequent step. It is not present in an overall reaction, and is present first as a product and then a reactant in the reaction mechanism.
- molecularity
- the number of reactant species (atoms, molecules, or ions) in an elementary reaction
- reaction mechanism (or path)
- the process, or pathway, by which a reaction occurs at the molecular level
- termolecular reaction
- the simultaneous collision of three atoms, molecules, or ions to produce one or more products
- unimolecular reaction
- a reaction with a rearrangement of a single reactant species to produce one or more products
Chemistry End of Section Exercises
- In general, can we predict the effect of doubling the concentration of A on the rate of the overall reaction A + B → C?
- Why are elementary reactions involving three or more reactants very uncommon?
- Write the rate equation for each of the following elementary reactions:
- O3(g) → O2(g) + O(g)
- O3(g) + Cl(g) → O2(g) + OCl(g)
- ClO(g) + O(g) → Cl(g) + O2(g)
- O3(g) + NO(g) → NO2(g) + O2(g)
- NO2(g) + O(g) → NO(g) + O2(g)
- Consider the following balanced equation:
NO(g) + O3(g) → NO2(g) + O2(g)
What is the rate law for this overall reaction?
- Rate = k[NO][O3]2
- Rate = k[NO2][O2]
- Rate = k[NO][O3]
- Rate = k[NO]2
- There is insufficient information to answer this question.
Answers to Chemistry End of Section Exercises
- No. In general, for the overall reaction, we cannot predict the effect of changing the concentration without knowing the rate law.
- The probability of three particles colliding simultaneously is very, very small.
- (a) Rate1 = k[O3]
(b) Rate2 = k[O3][Cl]
(c) Rate3 = k[ClO][O]
(d) Rate2 = k[O3][NO]
(e) Rate3 = k[NO2][O] - E
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

