M4Q4: Empirical Formula
Introduction
The section explores empirical formulas and the use of combustion to analyze a compound. This section includes worked examples, sample problems, and a glossary.
Learning Objectives for Empirical Formulas
- Determine empirical and chemical formulas using composition by mass, mass spectrometry, combustion analysis data, and molar mass information.
| Determination of Empirical Formulas | Derivation of Molecular Formulas | Mass Spectrometry of Molecules | Combustion Analysis |
| Key Concepts and Summary | Key Equations | Glossary | End of Section Exercises |
Determination of Empirical Formulas
As previously mentioned, the most common approach to determining a compound’s chemical formula is to first measure the masses of its constituent elements. However, we must keep in mind that chemical formulas represent the relative numbers, not masses, of atoms in the substance. Therefore, any experimentally derived data involving mass must be used to derive the corresponding numbers of atoms in the compound. To accomplish this, we can use molar masses to convert the mass of each element to number of moles. We then consider the moles of each element relative to each other, converting these numbers into a whole-number ratio that can be used to derive the empirical formula of the substance. Consider a sample of compound determined to contain 1.71 g C and 0.287 g H. The corresponding numbers of atoms (in moles) are:
1.71 g C × ![]() = 0.142 mol C
= 0.142 mol C
0.287 g H × ![]() = 0.285 mol H
= 0.285 mol H
Thus, we can accurately represent this compound with the formula C0.142H0.285. Of course, per accepted convention, formulas contain whole-number subscripts, which can be achieved by dividing each subscript by the smaller subscript:
![]() or CH2
or CH2
(Recall that subscripts of “1” are not written but rather assumed if no other number is present.)
The empirical formula for this compound is thus CH2. This may or not be the compound’s molecular formula as well; however, we would need additional information to make that determination (as discussed later in this section).
Consider as another example a sample of compound determined to contain 5.31 g Cl and 8.40 g O. Following the same approach yields a tentative empirical formula of:
Cl0.150O0.525 = ![]() = ClO3.5
= ClO3.5
In this case, dividing by the smallest subscript still leaves us with a decimal subscript in the empirical formula. To convert this into a whole number, we must multiply each of the subscripts by two, retaining the same atom ratio and yielding Cl2O7 as the final empirical formula.
In summary, empirical formulas are derived from experimentally measured element masses by:
- Deriving the number of moles of each element from its mass
- Dividing each element’s molar amount by the smallest molar amount to yield subscripts for a tentative empirical formula
- Multiplying all coefficients by an integer, if necessary, to ensure that the smallest whole-number ratio of subscripts is obtained
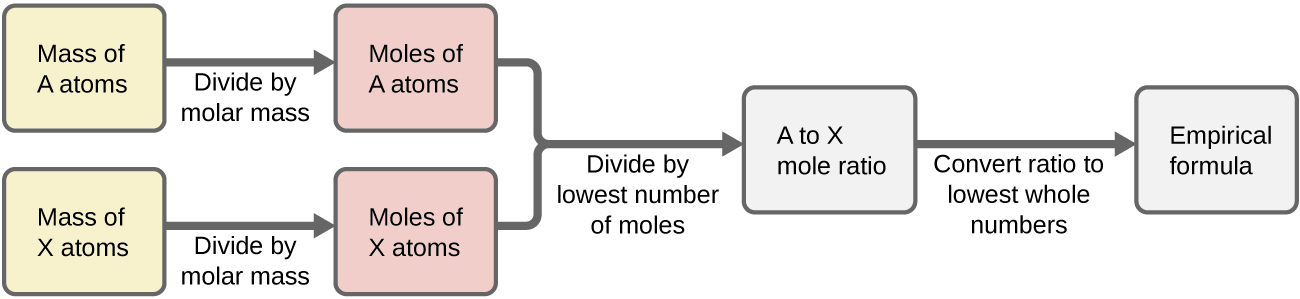
Figure 1 outlines this procedure in flow chart fashion for a substance containing elements A and X.
Example 1
Determining a Compound’s Empirical Formula from the Masses of Its Elements
A sample of the black mineral hematite (Figure 2), an oxide of iron found in many iron ores, contains 34.97 g of iron and 15.03 g of oxygen. What is the empirical formula of hematite?

Solution
For this problem, we are given the mass in grams of each element. Begin by finding the moles of each:
34.97 g Fe × ![]() = 0.6261 mol Fe
= 0.6261 mol Fe
15.03 g O × ![]() = 0.9394 mol O
= 0.9394 mol O
Next, derive the iron-to-oxygen molar ratio by dividing by the lesser number of moles:
![]() = 1.000 mol Fe
= 1.000 mol Fe
![]() = 1.500 mol O
= 1.500 mol O
The ratio is 1.000 mol of iron to 1.500 mol of oxygen (Fe1O1.5). Finally, multiply the ratio by two to get the smallest possible whole number subscripts while still maintaining the correct iron-to-oxygen ratio:
2 × Fe1O1.5 = Fe2O3
The empirical formula is Fe2O3.
Check Your Learning
What is the empirical formula of a compound if a sample contains 0.130 g of nitrogen and 0.370 g of oxygen?
Answer:
N2O5

For additional worked examples illustrating the derivation of empirical formulas, watch the brief video clip.
Deriving Empirical Formulas from Percent Composition
Finally, with regard to deriving empirical formulas, consider instances in which a compound’s percent composition is available rather than the absolute masses of the compound’s constituent elements. In such cases, the percent composition can be used to calculate the masses of elements present in any convenient mass of compound; these masses can then be used to derive the empirical formula in the usual fashion. Since the scale for percentages is 100, it is most convenient to assume a 100 g sample to calculate the mass of each element.
Example 2
Determining an Empirical Formula from Percent Composition
The bacterial fermentation of grain to produce ethanol forms a gas with a percent composition of 27.29% C and 72.71% O (Figure 3). What is the empirical formula for this gas?

Solution
Since the scale for percentages is 100, it is most convenient to calculate the mass of elements present in a sample weighing 100 g. The calculation is “most convenient” because, per the definition for percent composition, the mass of a given element in grams is numerically equivalent to the element’s mass percentage. This numerical equivalence results from the definition of the “percentage” unit, whose name is derived from the Latin phrase per centum meaning “by the hundred.” Considering this definition, the mass percentages provided may be more conveniently expressed as fractions:
27.29% C = ![]()
72.71% O = ![]()
The molar amounts of carbon and hydrogen in a 100-g sample are calculated by dividing each element’s mass by its molar mass:
27.29% C × ![]() = 2.272 mol C
= 2.272 mol C
72.71% O × ![]() = 4.544 mol O
= 4.544 mol O
Coefficients for the tentative empirical formula are derived by dividing each molar amount by the lesser of the two:
![]() = 1 C
= 1 C
![]() = 2 O
= 2 O
Since the resulting ratio is one carbon to two oxygen atoms, the empirical formula is CO2.
Check Your Learning
What is the empirical formula of a compound containing 40.0% C, 6.71% H, and 53.28% O?
Answer:
CH2O
Derivation of Molecular Formulas
Recall that empirical formulas are symbols representing the relative numbers of a compound’s elements. Determining the absolute numbers of atoms that compose a single molecule of a covalent compound requires knowledge of both its empirical formula and its molecular mass or molar mass. These quantities may be determined experimentally by various measurement techniques. Molecular mass, for example, is often derived from the mass spectrum of the compound (see discussion of this technique in the section on mass spectrometry of molecules from earlier in the semester). Molar mass can be measured by a number of experimental methods, many of which will be introduced in later chapters of this text.
Molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. As the name suggests, an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula. If we know the molecular (or molar) mass of the substance, we can divide this by the empirical formula mass in order to identify the number of empirical formula units per molecule, which we designate as n:
![]() = n formula units/molecule
= n formula units/molecule
The molecular formula is then obtained by multiplying each subscript in the empirical formula by n, as shown by the generic empirical formula AxBy:
(AxBy)n = AnxBny
For example, consider a covalent compound whose empirical formula is determined to be CH2O. The empirical formula mass for this compound is approximately 30 amu (the sum of 12 amu for one C atom, 2 amu for two H atoms, and 16 amu for one O atom). If the compound’s molecular mass is determined to be 180 amu, this indicates that molecules of this compound contain six times the number of atoms represented in the empirical formula:
![]() = 6 formula units/molecule
= 6 formula units/molecule
Molecules of this compound are then represented by molecular formulas whose subscripts are six times greater than those in the empirical formula:
(CH2O)6 = C6H12O6
Note that this same approach may be used when the molar mass (g/mol) instead of the molecular mass (amu) is used. In this case, we are merely considering one mole of empirical formula units and molecules, as opposed to single units and molecules.
Example 3
Determination of the Molecular Formula for Nicotine
Nicotine, an alkaloid in the nightshade family of plants that is mainly responsible for the addictive nature of cigarettes, contains 74.02% C, 8.710% H, and 17.27% N. If 40.57 g of nicotine contains 0.2500 mol nicotine, what is the molecular formula?
Solution
Determining the molecular formula from the provided data will require comparison of the compound’s empirical formula mass to its molar mass. As the first step, use the percent composition to derive the compound’s empirical formula. Assuming a convenient, a 100-g sample of nicotine yields the following molar amounts of its elements:
(74.02 g C)![]() = 6.163 mol C
= 6.163 mol C
(8.710 g H)![]() = 8.624 mol H
= 8.624 mol H
(17.27 g N)![]() = 1.233 mol N
= 1.233 mol N
Next, we calculate the molar ratios of these elements relative to the least abundant element, N.
![]() ≅ 5
≅ 5
![]() ≅ 7
≅ 7
![]() ≅ 1
≅ 1
The C-to-N and H-to-N molar ratios are adequately close to whole numbers, and so the empirical formula is C5H7N. The empirical formula mass for this compound is therefore 81.13 amu/formula unit, or 81.13 g/mol formula unit.
We calculate the molar mass for nicotine from the given mass and molar amount of compound:
![]()
Comparing the molar mass and empirical formula mass indicates that each nicotine molecule contains two formula units:
![]() = 2 formula units/molecule
= 2 formula units/molecule
Thus, we can derive the molecular formula for nicotine from the empirical formula by multiplying each subscript by two:
(C5H7N)2 = C10H14N2
Check Your Learning
What is the molecular formula of a compound with a percent composition of 49.47% C, 5.201% H, 28.84% N, and 16.48% O, and a molecular mass of 194.2 amu?
Answer:
C8H10N4O2
Mass Spectrometry
Previously, we have learned about how mass spectrometry can provide important information about the natural abundance of isotopes. Mass spectrometry can also provide experimental data on the empirical and molecular formula of a molecular substance.
Figure 4 and Figure 5 show the hard ionization and soft ionization mass spectrum of carbon dioxide, CO2. When all atom-to-atom bonds are broken in CO2 during hard ionization, the result is one C+ ion and two O+ ions.
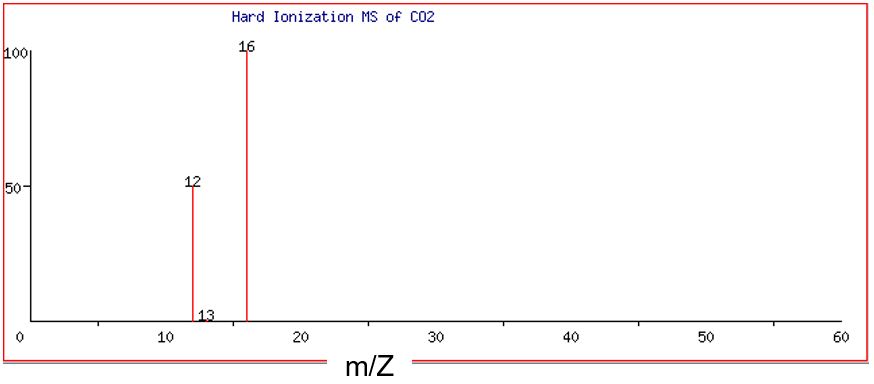
Note that there are three discrete peaks visible in the mass spectra of CO2: one major peak at 12 amu, one very small peak at 13 amu, and a major peak at 16 amu. (Remember that we are assuming that the charge, Z, is equal to +1. This means that if the peak is at m/Z = 12, then m must be 12 amu.) The peaks at 12 and 13 amu correspond to the two stable isotopes of the element carbon, 12C (98.9%) and 13C (1.1% ). Separate analysis shows that the element oxygen is composed of mainly the isotope 16O (99.76%), with just trace amounts of other isotopes. Thus, we can use the abundance of the two major peaks at 12 and 16 amu to obtain the ratio of carbon atoms to oxygen atoms in the molecule.
When subjected to soft ionization, the CO2 molecule remains intact, and ionization results in one CO2+ ion. Figure 5 shows that there are two discrete peaks visible in the mass spectra of CO2+ ion: one major peak at 44 amu and one very small peak at 45 amu.
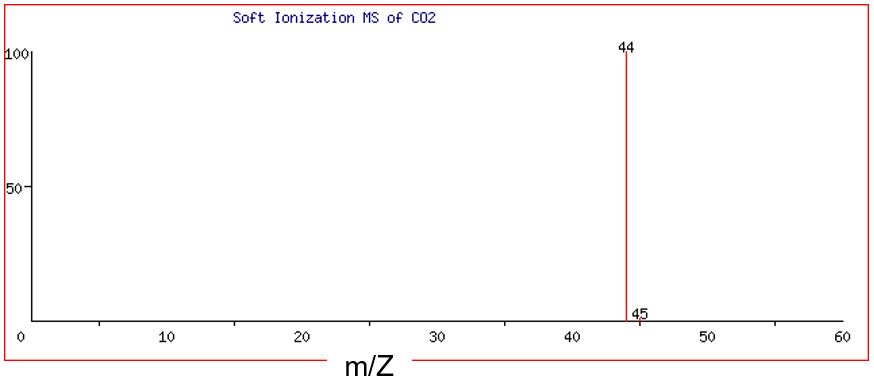
Processing the data from the hard MS and soft MS provides different information. From the hard MS, we can calculate the molar mass of the lowest ratio of atoms, i.e., the empirical formula. If there is one copy of carbon and two copies of oxygen, then the molecule has a molar mass of 44 amu (or 45 amu if the isotope of carbon in the molecule is the minor 13C). Since the molecule is not broken into its constituent parts in soft MS, the soft MS spectrum tells us the molecular weight of the molecule and therefore its molecular formula. The empirical formula mass derived from hard MS is the same value as the major peak in the soft MS, which tells us that the molecular formula is the same as the empirical formula, which is CO2.
The CO2+ ion at 44 amu contains one carbon-12 atom plus two oxygen-16 atoms, while the CO2+ ion at 45 amu contains one carbon-13 atom plus two oxygen-16 atoms. As the abundance of carbon-12 is significantly greater than that of carbon-13, we expect the abundance of the ion at 44 amu to be much greater than that of the ion at 45 amu. Separate analysis shows the abundance to be 989 : 11 like that of the isotopes of the element carbon.
Example 4
More Complicated Mass Spectra
Use these mass spectra data to determine the chemical formula of an unknown compound. Assume your sample contains only the predominant isotopes of the atoms.
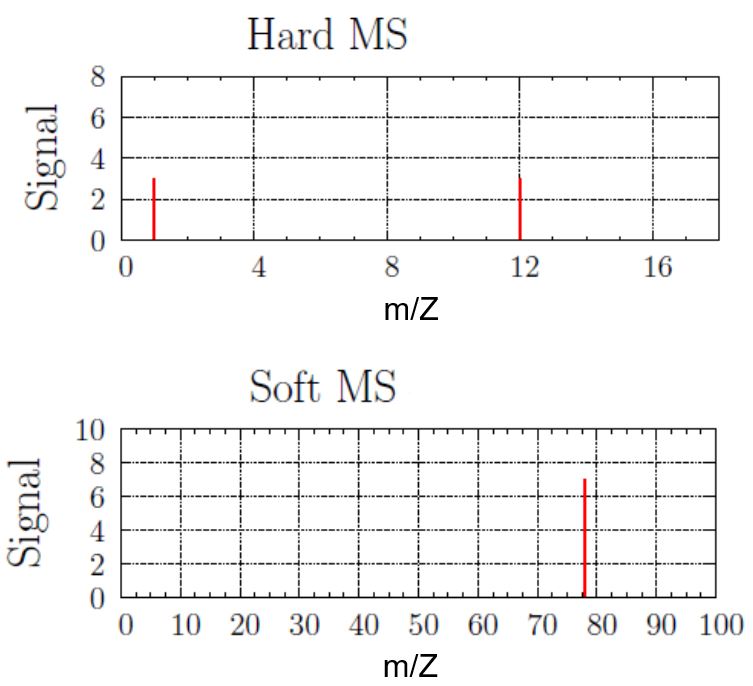
Solution
The hard MS has peaks at 1 amu and 12 amu, and they are approximately the same height. Hydrogen has mass of 1 amu and carbon has mass of 12 amu, and they are in a 1:1 ratio.
The soft MS has a single peak at 78 amu. If there was one hydrogen atom and one carbon atom in the molecule, then the mass of the compound would be 13 amu. However, there is no peak at 13 amu. There must be multiple copies of the CH-unit in the molecule. Dividing the molecular weight (78 amu) by the empirical formula’s molecular weight (13 amu) gives the number of copies. There are 78/13 or six copies of the CH unit. Thus, the unknown compound has a formula of C6H6.
Check Your Learning
Draw both the hard MS and soft MS of water.
Answer:
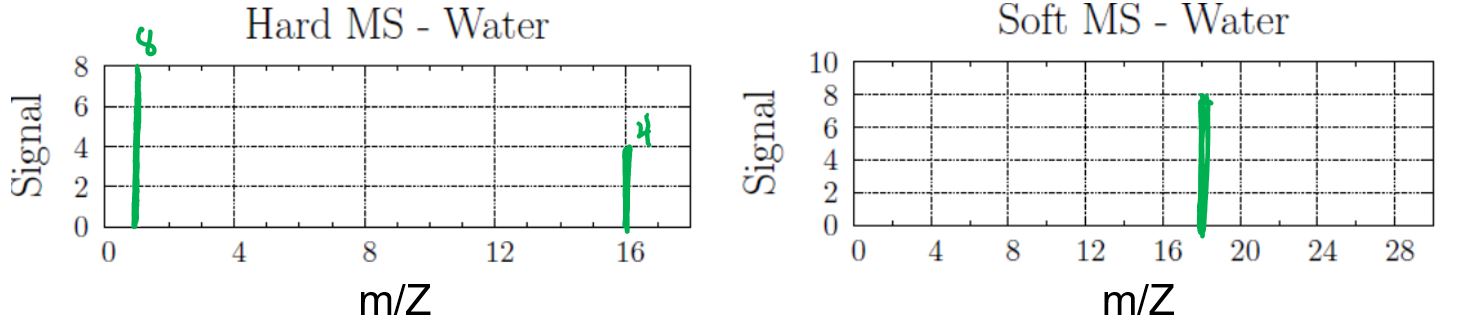
The formula for water is H2O.
In the hard MS, the signal at 1 amu represents H+ ion, and the signal at 16 amu represents O+ ion. The ratio of the abundance of H+ ion to O+ ion is 2:1. Note that as long as the two peaks in the hard MS are represented in a 2:1 ratio it would be correct, e.g., drawing a 6:3 H:O ratio would be equally acceptable.
In the soft MS, there is a single peak at 18 amu for the H2O+ ion (1 + 1 + 16 amu). The single peak can be drawn at any height.
Example 5
Mass Spectra of a Molecule Containing Bromine
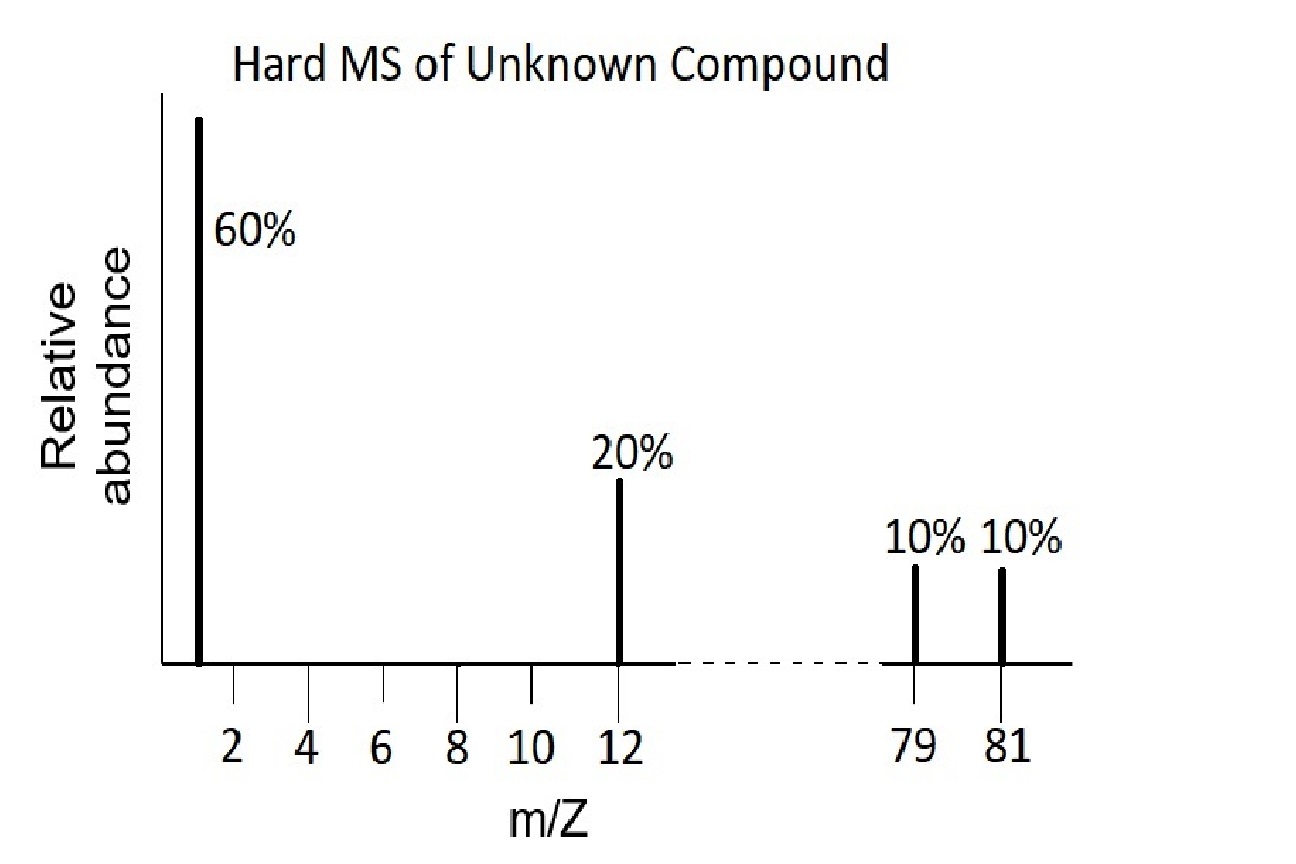
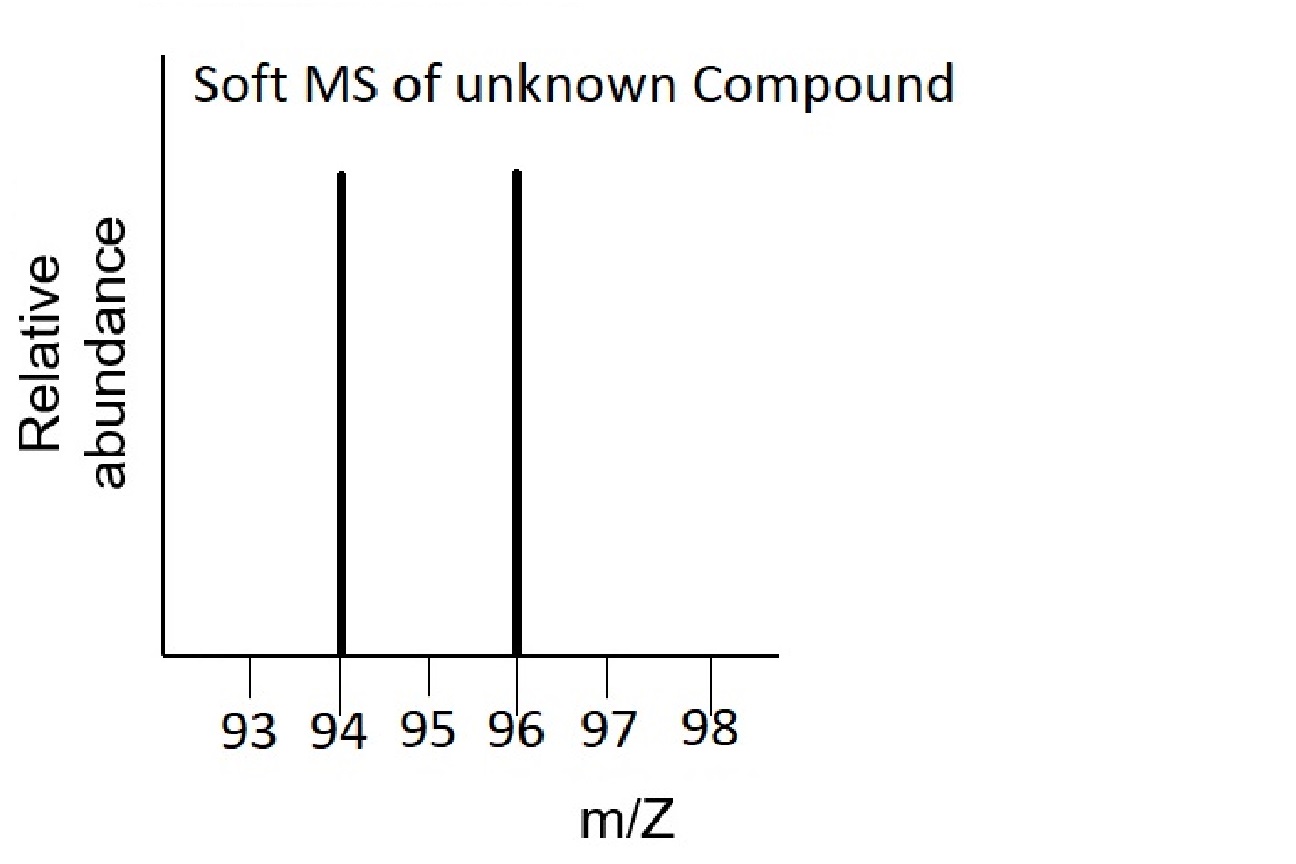
Use these mass spectra data to determine the chemical formula of an unknown compound that contains bromine. The element bromine consists of two isotopes, 79Br (50.7%) and 81Br (49.3%).
 |
 |
Solution
The hard MS has peaks at 1 amu, 12 amu, 79 amu and 81 amu. Hydrogen has mass of 1 amu and carbon has mass of 12 amu, and they are in a 3:1 ratio.
The two peaks at 79 and 81 amu correspond to the single element, Br, that exists as two isotopes in very nearly equal abundances. As a single element, the sum of the two percentages (10%+10%) needs to be calculated for comparison with the C and H. This indicates a C:H:Br ratio of 20:60:20 or 1:3:1. and an empirical formula of CH3Br.
The soft MS has two peaks at 94 and 96 corresponding to the molecular weights 94 amu and 96 amu of the two molecules CH379Br and CH381Br respectively. The empirical formula and actual formula are therefore the same for this molecule, i.e., CH3Br.
Combustion Analysis
The elemental composition of hydrocarbons and related compounds may also be determined via a method known as combustion analysis. In a combustion analysis, a weighed sample of the compound is heated to a high temperature under a stream of oxygen gas, resulting in its complete combustion to yield gaseous products of known identities. The complete combustion of hydrocarbons, for example, will yield carbon dioxide and water as the only products. The gaseous combustion products are swept through separate, preweighed collection devices containing compounds that selectively absorb each product (Figure 4). The mass increase of each device corresponds to the mass of the absorbed product and may be used in an appropriate stoichiometric calculation to derive the mass of the relevant element.
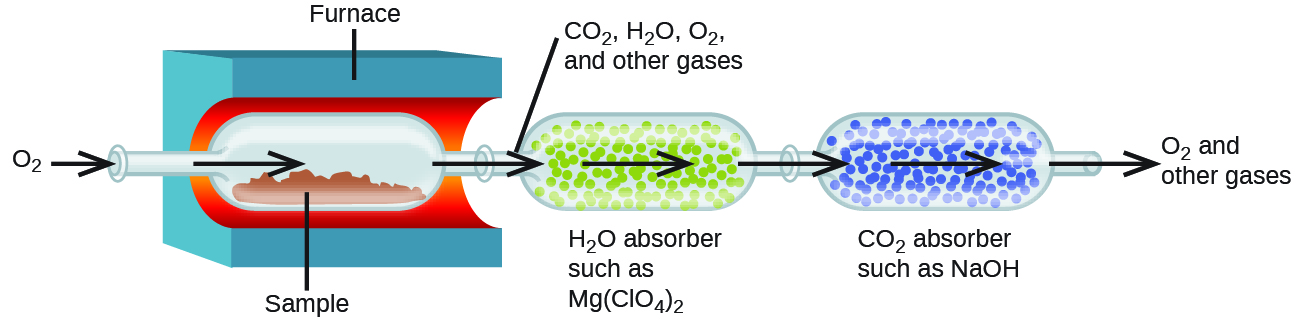
Example 6
Combustion Analysis
Polyethylene is a hydrocarbon (with formula CxHy) polymer used to produce food-storage bags and many other flexible plastic items. A combustion analysis of a 0.00126-g sample of polyethylene yields 0.00394 g of CO2 and 0.00161 g of H2O. What is the empirical formula of polyethylene?
Solution
The primary assumption in this exercise is that all the carbon in the sample combusted is converted to carbon dioxide, and all the hydrogen in the sample is converted to water:
CxHy(s) + excess O2(g) → x CO2(g) + ![]() H2O(g)
H2O(g)
Note that a balanced equation is not necessary for the task at hand. To derive the empirical formula of the compound, only the subscripts x and y are needed.
First, calculate the molar amounts of carbon and hydrogen in the sample, using the provided masses of the carbon dioxide and water, respectively. With these molar amounts, the empirical formula for the compound may be written as described earlier. An outline of this approach is given in the following flow chart:
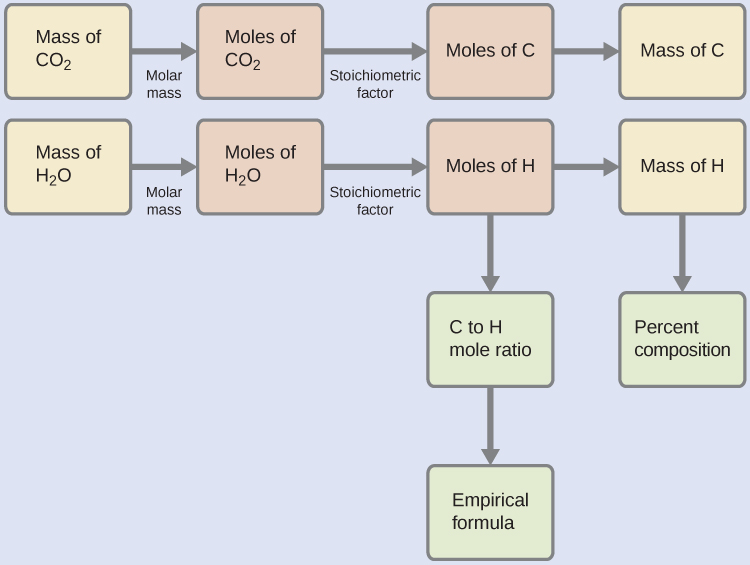
mol C = 0.00394 g CO2 × ![]() ×
× ![]() = 8.95 × 10-5 mol C
= 8.95 × 10-5 mol C
mol H = 0.00161 g H2O × ![]() ×
× ![]() = 1.79 × 10-4 mol H
= 1.79 × 10-4 mol H
The empirical formula for the compound is then derived by identifying the smallest whole-number multiples for these molar amounts. The H-to-C molar ratio is
![]() =
= ![]() =
= ![]()
and the empirical formula for polyethylene is CH2.
Check Your Learning
A 0.00215-g sample of polystyrene, a polymer composed of carbon and hydrogen, produced 0.00726 g of CO2 and 0.00148 g of H2O in a combustion analysis. What is the empirical formula for polystyrene?
Answer:
CH
Example 7
Combustion Analysis of an Organic Compound Containing Oxygen
Combustion analysis of 23.1 mg of a white powder that contains only the elements C, H, and O yields 33.9 mg of CO2 and 13.9 mg of H2O. Determine the empirical formula of the white compound.
Solution
The mass of C and H in the white powder that produced the quantities of CO2 and H2O in the analysis must first be determined. This enables the amount of O in the compound to be calculated by subtraction, as this oxygen does not react in the combustion process.:
mass of C = 33.9 mg CO2 × ![]() ×
× ![]() ×
× ![]() ×
× ![]() ×
× ![]() = 9.25 mg C
= 9.25 mg C
mass of H = 13.9 mg H2O × ![]() ×
× ![]() ×
× ![]() ×
× ![]() ×
× ![]() = 1.54 mg H
= 1.54 mg H
Because the white powder consists of just three elements and we now know the mass of two of them, the mass of O is the remainder:
mass O = 23.1 mg – (9.25 mg + 1.54 mg) = 12.3 mg O
Now the moles of each element can be determined, and the empirical formula determined:
moles C: 9.25 mg C × ![]() ×
× ![]() = 7.70 × 10-4 mol C
= 7.70 × 10-4 mol C
moles H: 1.54 mg H × ![]() ×
× ![]() = 1.53 × 10-3 mol H
= 1.53 × 10-3 mol H
moles O: 12.3 mg O × ![]() ×
× ![]() = 7.69 × 10-4 mol O
= 7.69 × 10-4 mol O
Dividing each of these by the smallest number of moles (7.69 x 10-4 mol) yields the empirical formula: CH2O
This approach is outlined in the following flow chart:
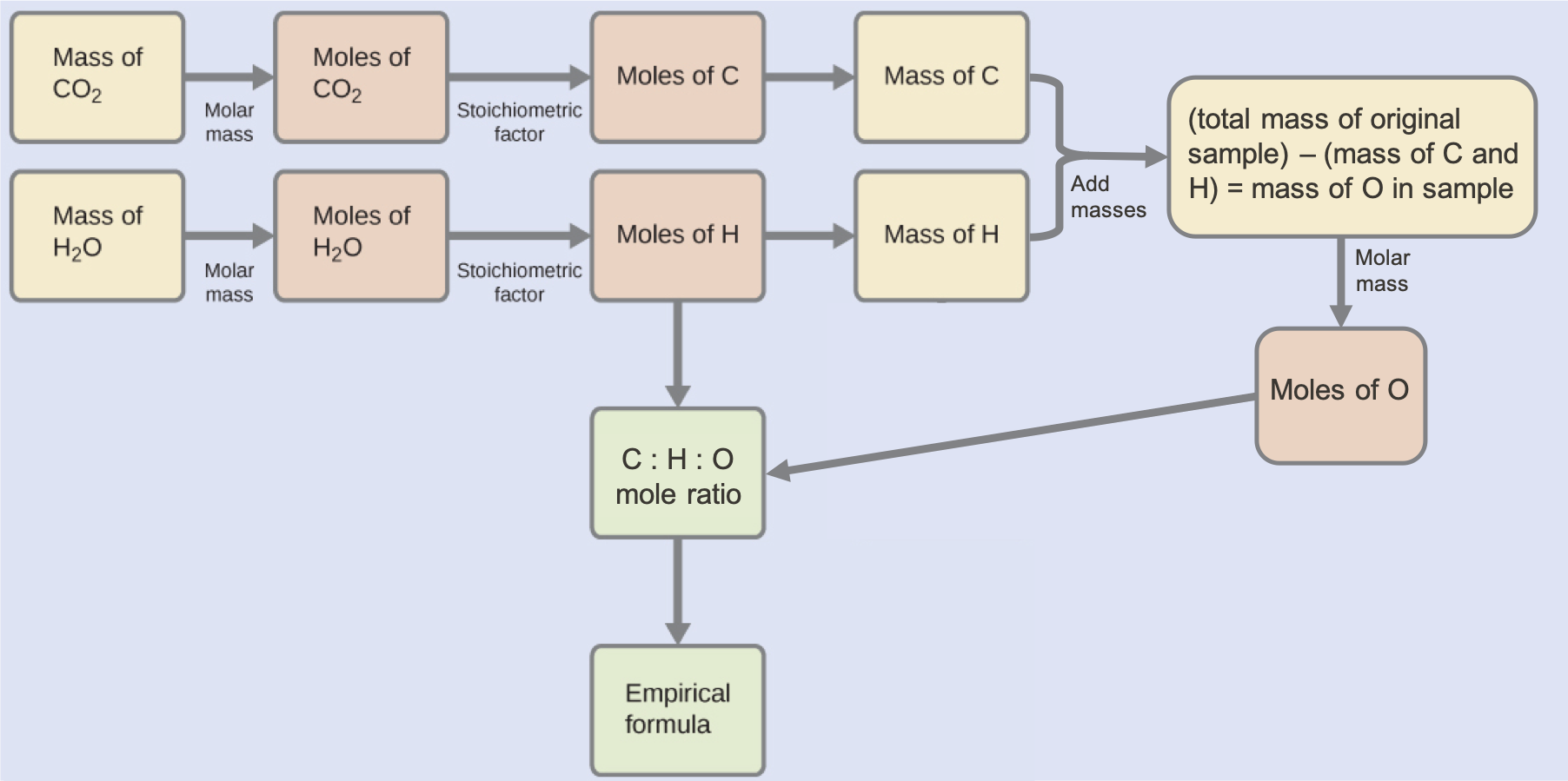
Check Your Learning
Combustion analysis of 0.112 g of a compound that contains C, H, and O yields 0.0561 g of H2O and 0.205 g of CO2. The hard mass spectrum shows three peaks at 1, 12, and 16 amu, and the soft mass spectrum a single peak at 144 amu. Determine the empirical and actual formula of the compound.
Answer:
The hard mass spectrum indicates the compound consists of the elements C, H, and O.
Empirical Formula C3H4O2, actual Formula C6H8O4
Key Concepts and Summary
The chemical identity of a substance is defined by the types and relative numbers of atoms composing its fundamental entities. To determine an empirical formula, you must find the smallest whole-number ratio of atoms in a molecule. If you begin with the mass of each element in the compound, you must first convert these masses to moles before determining the ratio. A compound’s percent composition provides the mass percentage of each element in the compound, and it is often experimentally determined and used to derive the compound’s empirical formula. The empirical formula mass of a covalent compound may be compared to the compound’s molecular or molar mass to derive a molecular formula. Combustion analysis can be used to identify an unknown hydrocarbon because the identity of the products are known to be CO2 and H2O.
Key Equations
- %X =
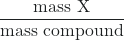 × 100%
× 100% 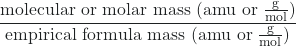 = n formula units/molecule
= n formula units/molecule- (AxBy)n = AnxBny
Glossary
- combustion analysis
- analytical technique used to determine the elemental composition of a compound via the collection and weighing of its gaseous combustion products
- empirical formula
- formula showing the composition of a compound given as the simplest whole-number ratio of atoms
- empirical formula mass
- sum of average atomic masses for all atoms represented in an empirical formula
- molecular formula
- formula indicating the composition of a molecule of a compound and giving the actual number of atoms of each element in a molecule of the compound
- percent composition
- percentage by mass of the various elements in a compound
Chemistry End of Section Exercises
- What information do we need to determine the molecular formula of a compound from the empirical formula?
- Calculate the following to four significant figures:
- the percent composition of ammonia, NH3
- the percent composition of photographic “hypo,” Na2S2O3
- the percent of calcium ion in Ca3(PO4)2
- Determine the percent ammonia, NH3, in Co(NH3)6Cl3, to three significant figures.
- Determine the empirical formulas for compounds with the following percent compositions:
- 15.8% carbon and 84.2% sulfur
- 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen
- A compound contains carbon, hydrogen, and chlorine. It has a molar mass of 99 g/mol. Analysis of a sample shows that it contains 24.3% carbon and 4.1% hydrogen. What is its molecular formula?
- Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol.
- Polymers are large molecules composed of simple units repeated many times. Thus, they often have relatively simple empirical formulas. Calculate the empirical formulas of the following polymers:
- Lucite (Plexiglas); 59.9% C, 8.06% H, 32.0% O
- Saran; 24.8% C, 2.0% H, 73.1% Cl
- polyethylene; 86% C, 14% H
- polystyrene; 92.3% C, 7.7% H
- Orlon; 67.9% C, 5.70% H, 26.4% N
- A major textile dye manufacturer developed a new yellow dye. The dye has a percent composition of 75.95% C, 17.72% N, and 6.33% H by mass with a molar mass of about 240 g/mol. Determine the molecular formula of the dye.
- Deduce the empirical formula (element ratio) from the following hard ionization mass spectrum:
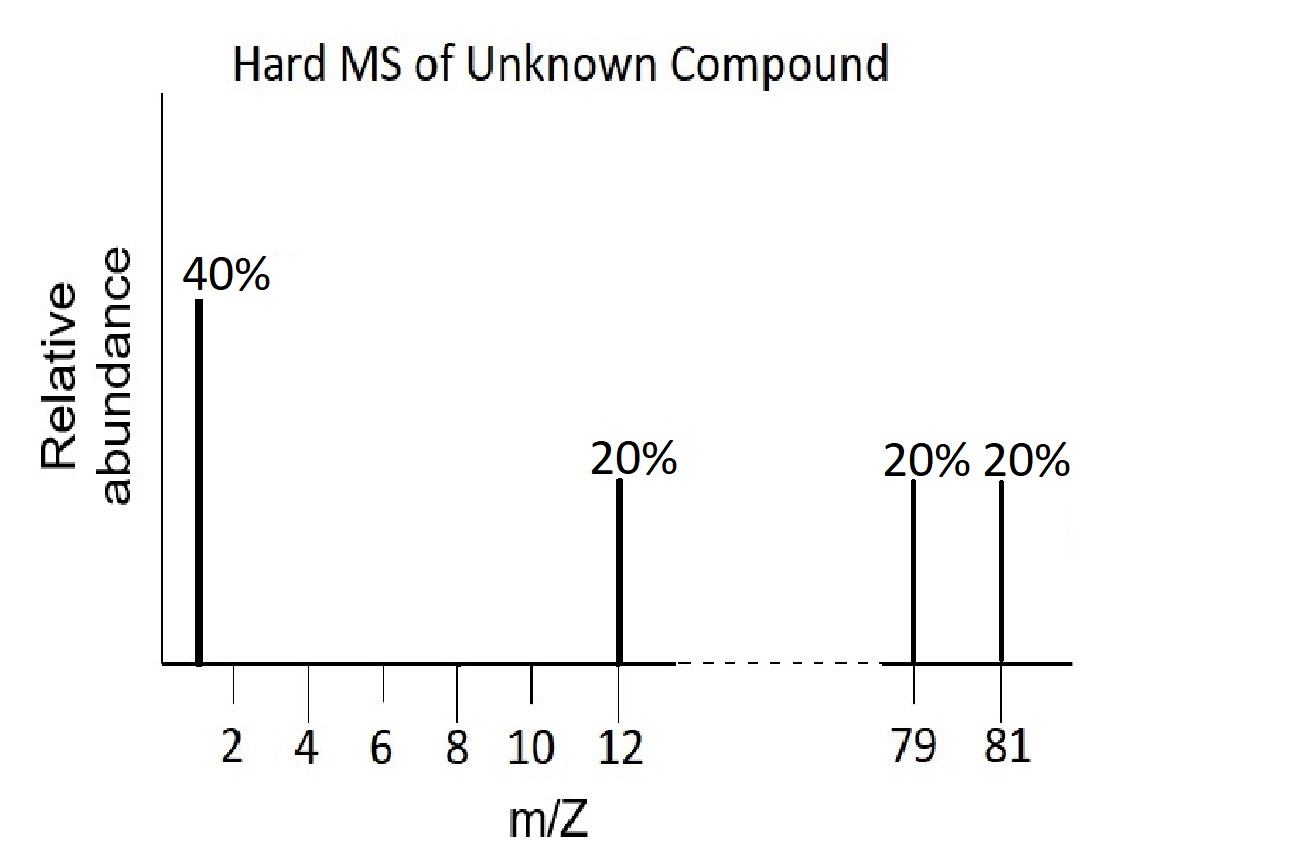
Given that the empirical formula for the compound is the same as the actual molecular formula, draw a representation of the soft ionization mass spectrum for this compound.
- The hard and soft MS of a particular compound are given below. In a hard MS, all bonds in a molecule are broken and individual atoms are observed. In a soft MS, no bonds are broken, and the molecule is observed.
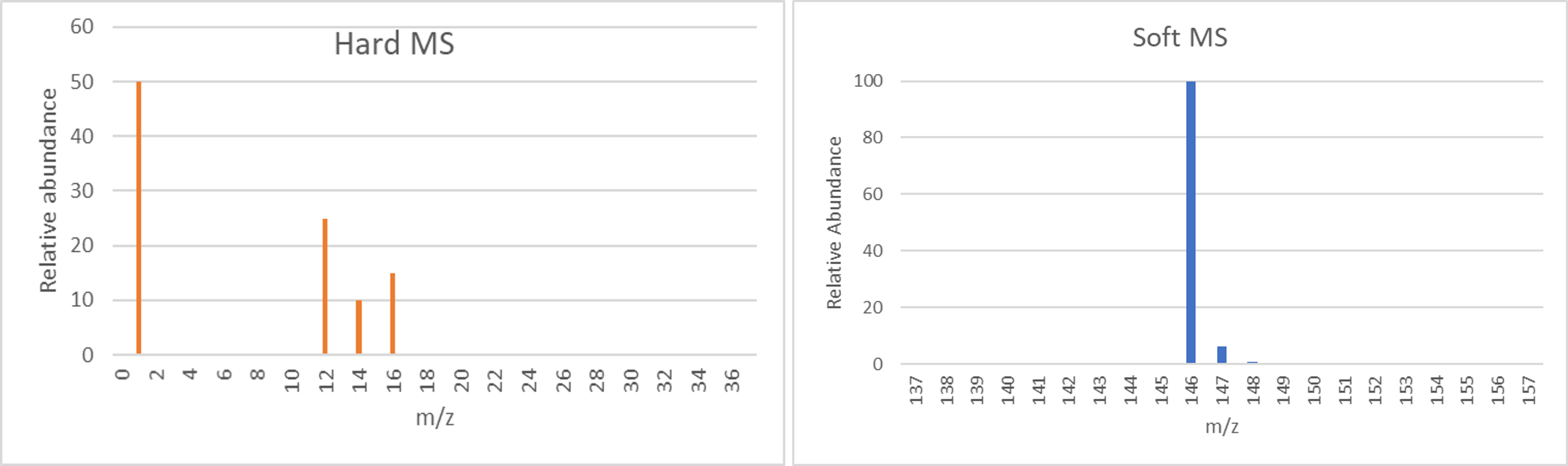
- Based on the hard MS, what elements are present in this compound?
- Based on the hard MS, what is the empirical formula of this compound?
- Based on both the hard and soft MS, what is the molecular formula of this compound?
- Draw both the hard and soft mass spectrum for phosphorus trifluoride. Both P and F are monotopic, i.e., exist as single isotopes.
- A 0.025-g sample of a compound composed of boron and hydrogen, with a molecular mass of ~28 amu, burns spontaneously when exposed to air, producing 0.063 g of B2O3. What are the empirical and molecular formulas of the compound? The general reaction is
BxHy + O2 → B2O3 + H2O (not balanced)
- A compound has a molecular mass of about 130 amu, and contains only carbon and hydrogen. A 3.000-mg sample of this compound burns to give 10.3 mg of CO2. Determine its empirical and molecular formulas.
- Combustion analysis of 0.112 g of a compound that contains C, H, and O yields 0.0561 g of H2O and 0.205 g of CO2. The hard mass spectrum shows three peaks at 1, 12, and 16 amu, and the soft mass spectrum shows a single peak at 144 amu. Determine the empirical and actual formula of the compound.
Answers to Chemistry End of Section Exercises
- You need a given mass and the molar amount of the compound to get the molar mass so it can be compared to the empirical mass
- (a) % N = 82.24%, % H = 17.76%
(b) % Na = 29.08%, % S = 40.56%, % O = 30.36%
(c) % Ca2+ = 38.76% - % NH3 = 38.2%
- (a) CS2; (b) CH2O
- C2H4Cl2
Left-click here to watch Exercise 5 problem solving video.
- Mg3Si2H3O8 (empirical formula), Mg6Si4H6O16 (molecular formula)
- (a) C5H8O2; (b) CHCl; (c) CH2; (d) CH; (e) C3H3N
- C15H15N3
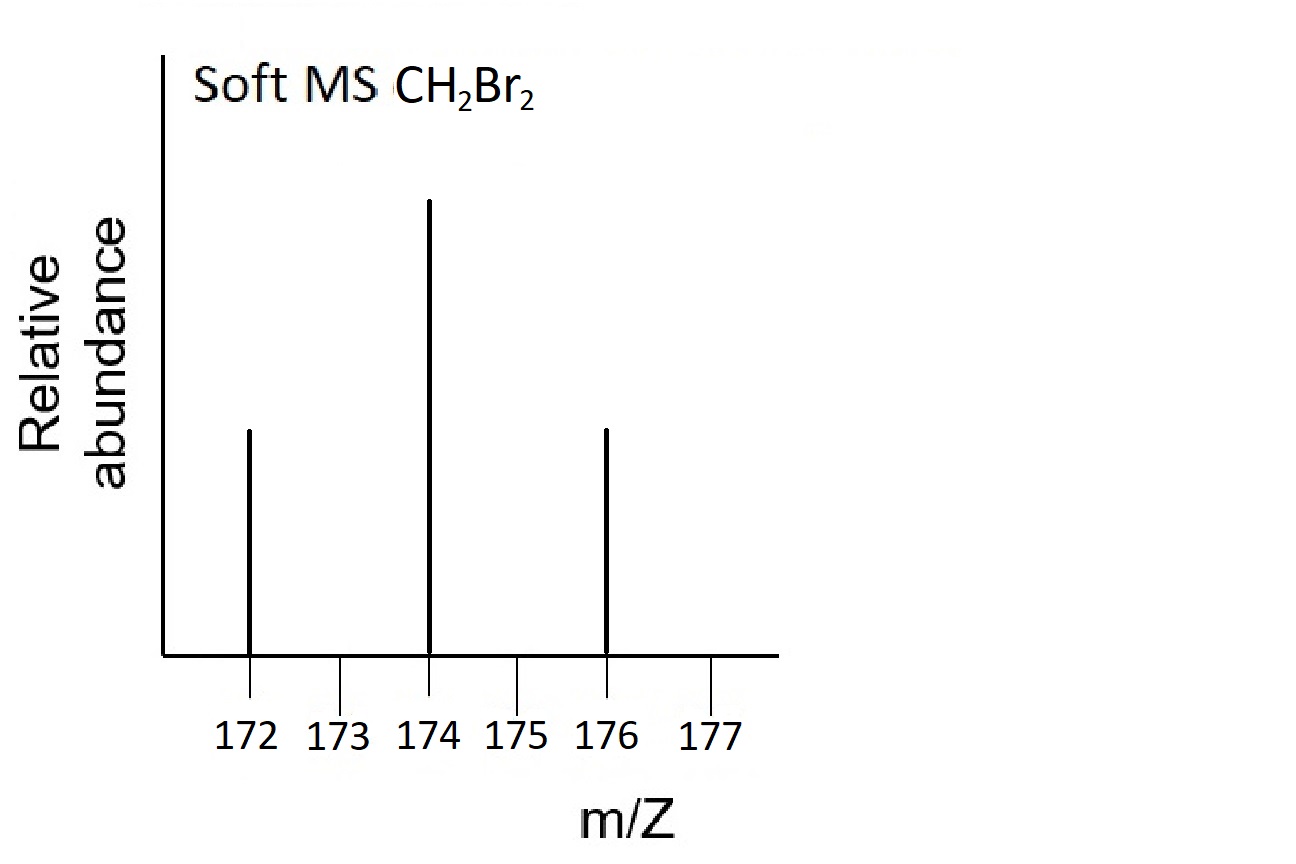
- CH2Br2. With two isotopes of Br and two Br atoms in each molecule there are four possible molecules: CH279Br79Br, CH279Br81Br, CH281Br79Br, and CH281Br81Br with masses of 172, 174, 174, and 176 amu respectively. The soft mass spectrum will therefore exhibit three peaks in a 1:2:1 ratio as two of the molecules have the same mass, which is twice as likely to occur as the other two possibilities:
- (a) H, C, N, O
(b) H10C5N2O3
(c) H10C5N2O3 - For hard MS, 1:3 relative abundance of P:F with peaks at m/Z = 19 (F) and m/Z = 31 (P). For soft MS, peak at m/Z = 88 (PF3) with 100% abundance.
- The empirical formula is BH3. The molecular formula is B2H6.
- Empirical formula: C5H4, molecular formula: C10H8
- The hard mass spectrum indicates the compound consists of the elements C, H, and O. Empirical formula: C3H4O2, molecular formula: C6H8O4
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

