M7Q10: Electron Configurations for Ions
Introduction
This section explores writing electron configurations for ions and describing trends in ionic radii. This section includes worked examples, a glossary, and practice problems.
Learning Objectives for Electron Configurations for Ions
- Correlate the effective nuclear charge with selected trends in periodic properties.
| Ionic Radii | Electron Configurations of Ions | - Analyze the electron configuration of salts to determine their magnetism.
| Magnetism of Salts |
| Key Concepts and Summary | Glossary | End of Section Exercises |
Ionic Radii
Ionic radius is the measure used to describe the size of an ion. A cation always has fewer electrons and the same number of protons as the parent atom; thus, it is smaller than the atom from which it is derived (Figure 1). For example, the radius of a neutral aluminum atom (1s22s22p63s23p1) is 118 pm, whereas the ionic radius of an Al3+ (1s22s22p6) is 68 pm. All electrons in the n=3 shell are removed, the remaining electrons occupying smaller shells. Even the removal of a single electron produces a cation that is smaller than the parent atom as more accurate methods of determining Zeff that account for the screening effect of the valence electrons, show that losing a valence electron increases Zeff for any remaining valence electrons.

Cations with larger charges are smaller than cations with smaller charges (e.g., V2+ has an ionic radius of 79 pm, while that of V3+ is 64 pm). Proceeding down the groups of the periodic table, we find that cations of successive elements with the same charge generally have larger radii, corresponding to an increase in the principal quantum number, n.
An anion is formed by the addition of one or more electrons to the valence shell of an atom. This results in a greater repulsion among the valence electrons and a decrease in Zeff per valence electron, as valence electrons do contribute to screening albeit to a much lesser degree than the core electrons. Both effects (the increased number of electrons and the decreased Zeff) cause the radius of an anion to be larger than that of the neutral atom (Figure 1). For example, a sulfur atom ([Ne]3s23p4) has a covalent radius of 104 pm, whereas the ionic radius of the sulfide anion ([Ne]3s23p6) is 170 pm. For consecutive elements proceeding down any group, anions have larger principal quantum numbers and, thus, larger radii.
Atoms and ions that have the same electron configuration are said to be isoelectronic. Examples of isoelectronic species are N3–, O2–, F–, Ne, Na+, Mg2+, and Al3+ (all have the electron configuration 1s22s22p6). Another isoelectronic series is P3–, S2–, Cl–, Ar, K+, Ca2+, and Sc3+ ([Ne]3s23p6). For atoms or ions that are isoelectronic, the number of protons determines the size. The greater the nuclear charge, the smaller the radius in a series of isoelectronic ions and atoms because the electrons on the atom with the greatest nuclear charge will experience the highest Zeff.
Electron Configurations of Ions
We have seen that ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed from a neutral atom. The outermost or valence electrons are removed since they have the highest energies, are shielded more, and are farthest from the nucleus. For main group elements, the electrons that were added last are the first electrons removed. For transition metals and inner transition metals, however, electrons in the s orbital are easier to remove than the d or f electrons, and so the highest ns electrons are lost, and then the (n – 1)d or (n – 2)f electrons are removed. An anion forms when one or more electrons are added to a neutral atom. The added electrons fill in the order predicted by the Aufbau principle as we learned in a previous section.
Example 1
Predicting Electron Configurations of Ions
What is the ground state electron configuration of:
- Na+
- P3–
- Al2+
- Fe2+
- Sm3+
Solution
First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also acceptable.
Next, determine whether an electron is gained or lost. Remember electrons are negatively charged, so ions with a positive charge have lost an electron. For main group elements, the last orbital gains or loses the electron. For transition metals, the last s orbital loses an electron before the d orbitals.
(a) Na: 1s22s22p63s1. Sodium cation loses one electron, so Na+: 1s22s22p6.
(b) P: 1s22s22p63s23p3. Phosphorus anion (phosphide) gains three electrons, so P3−: 1s22s22p63s23p6.
(c) Al: 1s22s22p63s23p1. Aluminum cation loses two electrons, so Al2+: 1s22s22p63s1.
(d) Fe: 1s22s22p63s23p64s23d6. Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital. Fe2+: 1s22s22p63s23p63d6.
(e) Sm: 1s22s22p63s23p64s23d104p65s24d105p66s24f6. Samarium cation loses three electrons. The first two will be lost from the 6s orbital, and the final one is removed from the 4f orbital. Sm3+: 1s22s22p63s23p64s23d104p65s24d105p64f5.
Check Your Learning
Which ion with a +2 charge has the electron configuration 1s22s22p63s23p63d104s24p64d5? Which ion with a 3+ charge has this configuration?
Answer:
Tc2+, Ru3+
Magnetism of Salts
Electron configuration of ions is important when analyzing the magnetism of salts. Hund’s Rule states that electrons must occupy every orbital singly before any orbital is doubly occupied. This may leave an ion with many unpaired electrons. Paramagnetic salts are composed of ions that have one or more unpaired electrons. Due to the magnetic dipole moments of the unpaired electrons, paramagnetic salts are attracted by a magnetic field, a characteristic called paramagnetism.
Experimentally, magnetic susceptibility measures the force experienced by a substance in a magnetic field. When we compare the weight of a sample to the weight measured in a magnetic field (Figure 2), paramagnetic samples that are attracted to the magnet will appear heavier because of the force exerted by the magnetic field.
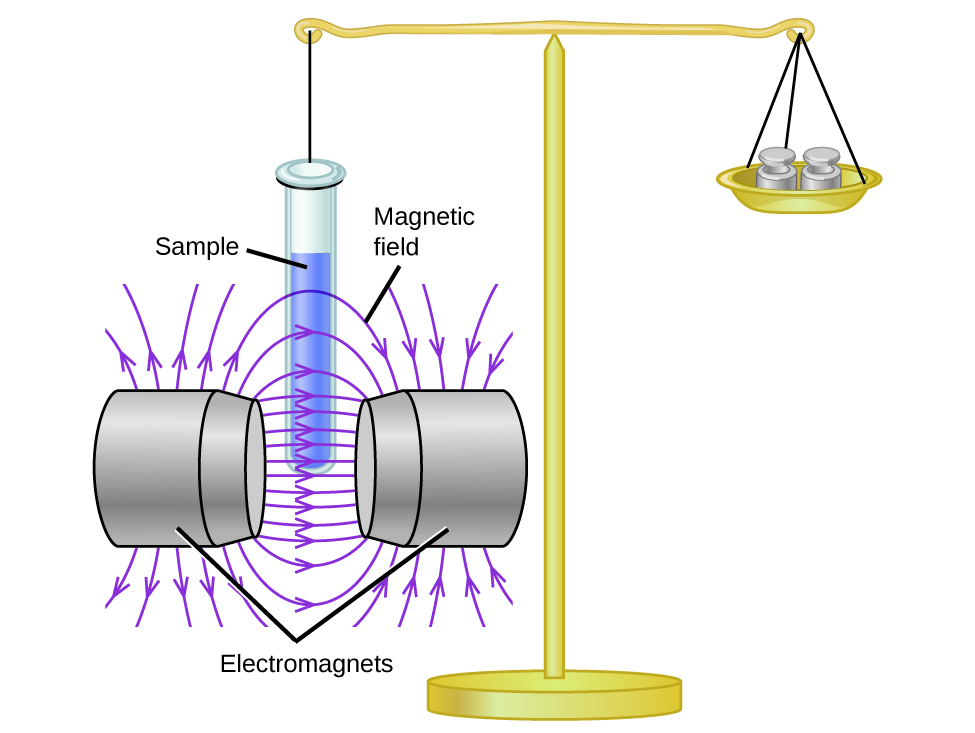
Diamagnetic salts are composed of ions that have only paired electrons, i.e., no unpaired electrons. According to the Pauli Exclusion Principle, which states that no two electrons may occupy the same quantum state at the same time, the electron spins of paired electrons are oriented in opposite directions. This causes the magnetic fields of the paired electrons to cancel out. Thus there is no net magnetic moment, and diamagnetic salts very weakly repel from a magnetic field, a characteristics called diamagnetism.
All substances exhibit diamagnetism, but because it is so weak, it is usually too small to notice. Substances exhibiting only diamagnetism are said to be diamagnetic. Some substances also exhibit paramagnetism, and are said to be paramagnetic. (You are probably more familiar with refrigerator magnets, iron magnets, or neodymium/rare-earth magnets. These exhibit ferromagnetism, a strong attraction to a magnetic field that is easily observable and can be made permanent. Unlike ferromagnetic substances, paramagnetic and diamagnetic substances do not act as permanent magnets.)
To determine whether an ion is paramagnetic or diamagnetic, you need to create and analyze the orbital box diagram to determine if there are unpaired electrons. For most salts, the anions in the salt are diamagnetic. Therefore, the magnetism of the salt is determined by its metal cations. By analyzing whether the metal cation has unpaired electrons, we can determine if the salt will be attracted to a magnet (i.e., be paramagnetic) or not (i.e., be diamagnetic).
Demonstration: Paramagnetic ions are attracted to a magnetic field
Set up. The following video shows how paramagnetic salts and diamagnetic salts react differently to a magnetic field. In this demonstration, the salts are contained in vials and a magnet is brought in from the right side of the screen. The ruler is used to show how close the magnet must get before the vial is attracted to the magnet (if at all). The salts shown are ZnSO4(s), Fe2O3(s), MnSO4(s), and FeCl3(s).
Prediction. Before watching the video, predict which of these salts will be attracted to the magnet.
Explanation. This demonstration shows that ZnSO4(s) is not attracted to the magnet and is thus diamagnetic. The Zn2+ cation is formed when the two 4s electrons are removed from the Zn atom, leaving the electron configuration [Ar]3d10, which contains all paired electrons. The other salts are all paramagnetic and attracted to the magnet. Fe3+ (in Fe2O3 and FeCl3) has the electron configuration [Ar]3d5, containing unpaired electrons. Mn2+ has the electron configuration [Ar]3d5, which also has unpaired electrons. These paramagnetic ions cause these salts to be attracted to the magnetic field.
Key Concepts and Summary
Anionic radii are larger than the neutral parent atom since there are more electrons yet the nuclear charge has remained constant, so the valence electrons experience a lower Zeff. Cationic radii are smaller than the neutral parent atom because the number of valence electrons has decreased while the nuclear charge has remained constant, leading to an increased Zeff and if all of the valence electrons are removed, the removal of an occupied outer shell. When determining the electron configuration of an ion, for main group elements, the electrons that were added last are the ones that are removed first when forming a cation. For transition metal elements, the ns electrons are easier to remove than the d or f electrons, so the s electrons are removed first.
Paramagnetic substances are weakly attracted to a magnetic field, while diamagnetic substances are not. This is because paramagnetic substances have at least one unpaired electron.
Glossary
- diamagnetism
- phenomenon in which a material is not magnetic itself but is repelled by a magnetic field; it occurs when there are only paired electrons present
- isoelectronic
- group of ions or atoms that have identical electron configurations
- paramagnetism
- phenomenon in which a material is not magnetic itself but is attracted to a magnetic field; it occurs when there are unpaired electrons present
Chemistry End of Section Exercises
- What additional information do we need to answer the question “Which ion has the electron configuration 1s22s22p63s23p6”?
- Thallium was used as a poison in the Agatha Christie mystery story “The Pale Horse.” Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron structure of the +1 cation of thallium.
- In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, Co2+ and Co3+. Write the electron structure of the two cations.
- Which ion with a +1 charge has the electron configuration 1s22s22p63s23p63d104s24p6? Which ion with a –2 charge has this configuration?
- Write the electron configurations for the following atoms or ions:
- B3+
- O–
- Cl3+
- Ca2+
- Ti
- The ground state electron configuration for a particular ion is [Kr]5s24d105p6. Select the identity of this ion.
- Sn3-
- Xe+
- Sb2+
- I–
- In+
- V3+ contains 2 valence electrons. Which of the following sets of quantum numbers could represent one of these two electrons?
- (4, 0, 0, +½)
- (3, 2, 0, -½)
- (3, 0, 0, –½)
- (3, 1, –1, +½)
- (4, 1, –1, –½)
- Based on their positions in the periodic table, list the following ions in order of increasing radius: K+, Ca2+, Al3+, Si4+.
- List the following ions in order of increasing radius: Li+, Mg2+, Br–, Te2–.
- Which atom and/or ion is/are isoelectronic with Br+: Se2+, Se, As–, Kr, Ga3+, Cl–?
- Which of the following atoms and ions is/are isoelectronic with S2+: Si4+, Cl3+, Ar, As3+, Si, Al3+?
- Compare both the numbers of protons and electrons present in each to rank the following ions in order of increasing radius: As3–, Br–, K+, Mg2+.
- The ionic radii of the ions S2–, Cl–, and K+ are 184, 181, 138 pm respectively. Explain why these ions have different sizes even though they contain the same number of electrons.
- In each of the following pairs, select the atom or ion with the smallest radius.
- Na and K
- Cl– and Ca2+
- O and O2–
- P and F
- True or False: The bromide ion (Br–) has a smaller ionic radius than the rubidium ion (Rb+).
- Consider V and V3+.
- Draw the ground state electron configurations for V and V3+. Use noble gas notation for core electrons and orbital box notation for valence electrons.
- Which species will be attracted to a magnet?
- An ion has 2+ charge and a mass of 49.9 amu. This ion has two electrons with n = 1, eight electrons with n = 2, and eleven electrons with n = 3. Assume the sample is uniform and composed of only one type of isotope. Answer the following questions about this ion and its neutral atom.
- What is its atomic number?
- What is the number of neutrons in its nucleus?
- What is the ground state electron configuration of the neutral atom?
- Is the ion paramagnetic or diamagnetic?
- Determine the mass, in grams, of 1.80 × 1024 atoms of this isotope.
Answers to Chemistry End of Section Exercises
- The charge of the ion.
- 1s22s22p63s23p63d104s24p64d105s25p66s24f145d10
- (a) Co2+: 1s22s22p63s23p63d7
(b) Co3+: 1s22s22p63s23p63d6 - Rb+, Se2−
- (a) 1s2
(b) 1s22s22p5;
(c) 1s22s22p63s23p2;
(d) 1s22s22p63s23p6;
(e) 1s22s22p63s23p64s23d2 - D
- B
- Si4+ < Al3+ < Ca2+ < K+
- Li+ < Mg2+ < Br– < Te2-
- Se, As−
- Cl3+, Si
- Mg2+ < K+ < Br– < As3–
- Even though these ions are isoelectronic, they have different effective nuclear charge. Since the cations have electrons removed from its valence shell, its effective nuclear charge increases therefore making the radius smaller. For anions, since there are electrons being added to the valence shell, the effective nuclear charge decreases therefore the radius increases.
- (a) Na; (b) Ca2+; (c) O; (d) F
- False
- (a) V:
![Ground state electron configurations for V. The core electrons are abbreviated with [Ar], valence electrons are shown as two paired electrons in 4s and two unpaired electrons in 3d.](https://wisc.pb.unizin.org/app/uploads/sites/557/2022/04/e_config_valence_V.png) V3+:
V3+: ![Ground state electron configurations for V3+. The core electrons are abbreviated with [Ar], valence electrons are shown as two unpaired electrons in the 3d orbital.](https://wisc.pb.unizin.org/app/uploads/sites/557/2022/04/e_config_valence_V3.png)
(b) Both V and V3+ - (a) 23
(b) 27
(c) 1s2 2s2 2p6 3s2 3p6 4s2 3d3
(d) paramagnetic
(e) 150 g
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

