M13Q1: Introduction to Kinetics; Concept of Reaction Rate
Learning Objectives
- Describe real-world examples of the importance of kinetics on chemical processes.
- Identify factors that affect the rates of chemical reactions.
| Concentration | Temperature | Surface Area | Catalyst |
| Key Concepts and Summary | Glossary | End of Section Exercises |
Introduction

The lizard in the photograph is not simply enjoying the sunshine or working on its tan. The heat from the sun’s rays is critical to the lizard’s survival. A warm lizard can move faster than a cold one because the chemical reactions that allow its muscles to move occur more rapidly at higher temperatures. In the absence of warmth, the lizard is an easy meal for predators.
From baking a cake to determining the useful lifespan of a bridge, rates of chemical reactions play important roles in our understanding of processes that involve chemical changes. When planning to run a chemical reaction, there are many questions to answer. The first is: “Will the reaction produce the desired products in useful quantities?” A second might be: “How rapidly will the reaction occur?” A third question often asked when investigating reactions in greater detail is: “What specific nanoscale, or molecular-level, processes take place as the reaction occurs?”
The study of chemical kinetics concerns the second and third questions—that is, the rate at which a reaction yields products and the nano-scale process by which a reaction occurs. In this chapter, we will examine the factors that influence the rates of chemical reactions, the mechanisms by which some reactions proceed, and the quantitative techniques used to determine and describe the rate at which reactions occur.
Concept of Reaction Rate
Chemical kinetics is the study of the speeds (or rates) of chemical reactions and the microscopic pathways by which the atoms and molecules are transformed from reactant to products.
A rate is a measure of how some property varies with time. Speed is a familiar rate that expresses the distance traveled by an object in a given amount of time. Wage is a rate that represents the amount of money earned by a person working for a given amount of time. For a chemical process, the reaction rate can be defined as the speed at which reactants are converted to products for a particular reaction.
Reaction rates are therefore determined by measuring the time-dependence of some property that can be related to reactant or product amounts. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. For reactions involving one or more colored substances, rates may be monitored via measurements of light absorption. For reactions involving aqueous electrolytes, rates may be measured via changes in a solution’s conductivity. The units used to express rate indicate the quantity that is changing: for example, M/min for concentration, atm/s for pressure, g/hour for mass, and so on.
Factors Affecting Reaction Rates
Factors that influence chemical reaction rates include the concentration of reactants, temperature, the physical state of reactants and their dispersion, the solvent, and the presence of a catalyst.
Although a balanced chemical equation for a reaction describes the quantitative relationships between the amounts of reactants present and the amounts of products that can be formed, it gives us no information about whether or how fast a given reaction will occur. This information is obtained by studying the chemical kinetics of a reaction, which depend on various factors: reactant concentrations, temperature, physical states and surface areas of reactants, and solvent and catalyst properties if either are present. By studying the kinetics of a reaction, chemists gain insights into how to control reaction conditions to achieve a desired outcome.
Concentration Effects
Two substances cannot react with each other unless their constituent particles (molecules, atoms, or ions) come into contact. If there is no contact, the reaction rate will be zero. Conversely, the more reactant particles that collide per unit time, the more often a reaction between them can occur. Consequently, the reaction rate usually increases as the concentration of the reactants increases.
Demonstration: Reaction Rate and Concentration Dependence
Set up. NaHSO3(aq) and starch are added to different concentrations of KIO3(aq). A reaction occurs between reduced iodine and starch which creates a dark blue complex.
Prediction. Predict the order of the reaction using the timing of the video to monitor the length of each reaction.
Explanation: Each reaction begins at 7 seconds in the video. The reaction with 0.04 M KIO3(aq) takes a total of 9 seconds, with 0.02 M takes 18 seconds, and with 0.01 M takes 42 seconds. As the concentration halves, the reaction takes about twice as long, indicating that this is a first order reaction.
Chemistry in Real Life: Concentration and Reaction rate
Marble and limestone, which consist primarily of calcium carbonate (CaCO3), deteriorate as a result of the reaction with the pollutant sulfur dioxide. The rate of this reaction depends on the amount of sulfur dioxide in the air. Sulfur dioxide combines with water vapor in the air to produce sulfurous acid in the following reaction:
SO2(g) + H2O(g) → H2SO3(aq)
Calcium carbonate reacts with sulfurous acid as follows:
CaCO3(s) + H2SO3(aq) → CaSO3(aq) + CO2(g) + H2O(ℓ)
In a polluted atmosphere where the concentration of sulfur dioxide is high, calcium carbonate deteriorates more rapidly than in less polluted air.

Temperature Effects
Increasing the temperature of a system increases the average kinetic energy of its constituent particles. As the average kinetic energy increases, the particles move faster and collide more frequently per unit time and possess greater energy when they collide. Both of these factors increase the reaction rate. Hence the reaction rate of nearly all reactions increases with increasing temperature. Conversely, the reaction rate of most reactions decreases with decreasing temperature. For example, refrigeration slows the rate of growth of bacteria in foods by decreasing the reaction rates of biochemical reactions that enable bacteria to reproduce.
Demonstration: Reaction Rate and Temperature Dependence
Set up. NaHSO3(aq) and starch are added to three solutions of 0.04 M KIO3(aq), the first at room temperature, the second at a much cooler temperature, and the third at a much warmer temperature. A reaction occurs between reduced iodine and starch which creates a dark blue complex.
Prediction. Predict which reaction you think will occur the most quickly. The most slowly?
Explanation: The higher the temperature, the higher the average kinetic energy within the system, and the faster the reaction will occur. This is also in agreement with the Arrhenius equation (which we will learn about later in this unit), where an increase in temperature results in an increase in the reaction rate. The reaction at the coolest temperature occurs the most slowly.
Phase and Surface Area Effects
In heterogeneous reactions, those with reactants in different phases (for example, between solid and liquid reactants or gas and liquid reactants), the reaction takes place only at the interface between the two phases. Hence, the rate of a reaction between two phases depends to a great extent on the surface contact between them. A finely divided solid will have more surface area available for reaction than an equal amount of the same substance in one large piece. Thus a liquid will react more rapidly with a finely divided solid than with a large piece of the same solid. The rates of two phase reactions may be increased by stirring, which increases the contact surface area and replenishes that surface with unreacted species. For example, while large pieces of iron react slowly with acids; finely divided iron reacts much more rapidly (Figure 1). Another example is that large pieces of wood smolder, smaller pieces burn rapidly, and saw dust burns explosively.
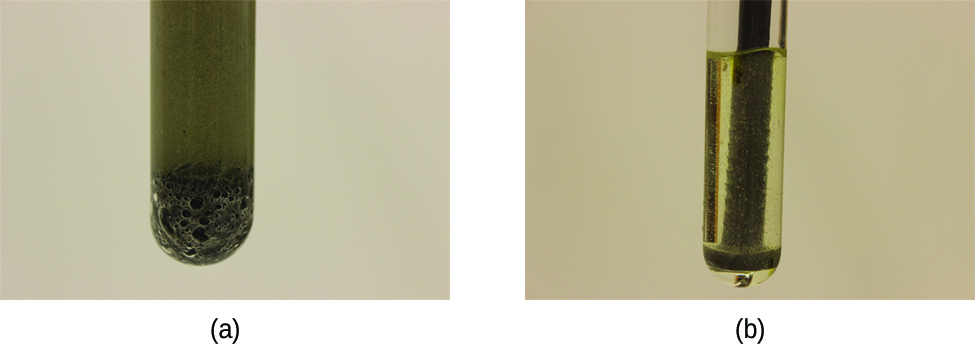
When an interface, or phase barrier, is not present between reactants, such as in a homogeneous gas mixture or in an aqueous solution, reactions occur much faster.
Demonstration: Reaction Rate and Particle Size
Set up. An iron bar and iron powder are each heated over an open flame. The amount of spark observed can be used as a measure of the extent of the reaction between iron and oxygen.
Prediction. Predict which reaction you think will occur the fastest.
Explanation: The reaction between the iron powder and oxygen creates the most spark. The iron bar creates no spark. The iron powder has much smaller particles, giving more surface area and increasing the rate of reaction.
Catalyst Effects
A catalyst is a substance that participates in a chemical reaction and increases the reaction rate without undergoing a net chemical change itself. Catalysts will be examined more closely later in this chapter.
Demonstration: Reaction Rate and Catalysts
Set up. A colorless solution of H2O2(aq) and NaKC4O6(aq) is warmed; however, there is no reaction. A small amount of CoCl2(aq) is added.
Prediction. Predict what will happen when the CoCl2 is added. Pay close attention to the color of the CoCl2 during the reaction. What role does CoCl2 play in the reaction?
Explanation: Once the CoCl2 is added, a vigorous bubbling reaction occurs. The initially pink CoCl2 turns green during the reaction, indicating a different cobalt-containing intermediate has been formed. The pink color is reestablished at the end of the reaction when CoCl2 is reformed. Because the CoCl2 is used in the reaction, but not consumed, it serves as a catalyst.
Demonstration: Reaction Rate and Catalysts
Set up. A colorless solution of H2O2(aq) and blue dishwashing liquid are mixed in a large graduated cylinder. Then, a yellow solution of KI(aq) is added.
Prediction. Predict what will happen when the KI is added. What role does KI play in the reaction?
Explanation: Once the KI is added, a sudden foaming reaction occurs. The KI serves as a catalyst during the reaction, increasing the rate of reaction.

Watch this video to see the reaction of cesium with water in slow motion and a discussion of how the state of reactants and particle size affect reaction rates.

Chemical reactions occur when molecules collide with each other and undergo a chemical transformation. Before physically performing a reaction in a laboratory, scientists can use molecular modeling simulations to predict how the parameters discussed earlier will influence the rate of a reaction. Use the PhET Reactions & Rates interactive to explore how temperature, concentration, and the nature of the reactants affect reaction rates.
Key Concepts and Summary
When studying the rate of a reaction and the nano-scale process (or mechanism) by which a reaction occurs, there are four primary categories that have an effect:
- Concentration – the more reactants there are, the higher the probability of collisions and products being formed
- Temperature – the higher the temperature, the more kinetic energy is available to the reactants, the higher the probability of collisions and products being formed
- Phase and Surface Area – Gas phase molecules move more quickly and freely in a reaction vessel, whereas liquids and solids are confined to the condensed phase. Within a liquid or solid phase, the greater the surface area, the greater the area for a reaction to occur, the higher the probability of a reaction and products being formed
- Catalyst – by definition, a catalyst increases the rate of the reaction without being consumed itself.
Glossary
- chemical kinetics
- the study of speeds (or rates) of chemical reactions and the microscopic pathways by which the atoms and molecules are transformed from reactants to products
- reaction rate
- the speed at which reactants are converted to products for a particular reaction, always a positive quantity
Chemistry End of Section Exercises
- What is the relationship between each of the following factors and the reaction rate: reactant concentration, temperature of the reaction, and the presence of a catalyst?
- Describe the effect of each of the following on the rate of the reaction of magnesium metal with a solution of hydrochloric acid:
- the molarity of the hydrochloric acid
- the temperature of the solution
- the size of the pieces of magnesium.
- If you were to make a glass of sweetened iced tea the old-fashioned way (by adding sugar and ice cubes to a glass of hot tea), which would you add first?
- Increasing pressure increases the rate of reaction for some reactions, particularly when one or more of the reactants are gaseous. Why might that be?
Answers to Chemistry End of Section Exercises
- Reaction rates generally increase with increasing reactant concentration, increasing temperature, and the addition of a catalyst.
- (a) Higher molarity increases the rate of the reaction.
(b) Higher temperature increases the rate of the reaction.
(c) Smaller pieces of magnesium metal will react more rapidly than larger pieces because more reactive surface exists. - The sugar will dissolve more rapidly at a higher temperature so add the sugar first, and then the ice.
- Increasing the pressure of a gaseous system decreases the volume so the reactants are in closer range of each other, will collide more often, and increase the rate of the reaction.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

