M2Q2: Rutherford’s Experiments
Introduction
This section explores Ernest Rutherford’s experiment that led to the understanding of the atom that we learned about in the previous section. We’ll provide background and context to Rutherford’s experiments. The section below provides description of these topics, worked examples, practice problems and a glossary of important terms.
Learning Objectives for Rutherford’s Experiments
- Distinguish between the size of the nucleus and the size of an electron cloud; recognize the significance of Rutherford’s experiment.
| Rutherford’s Experiment |
| Key Concepts and Summary | Glossary | End of Section Exercises |
Rutherford’s Experiment
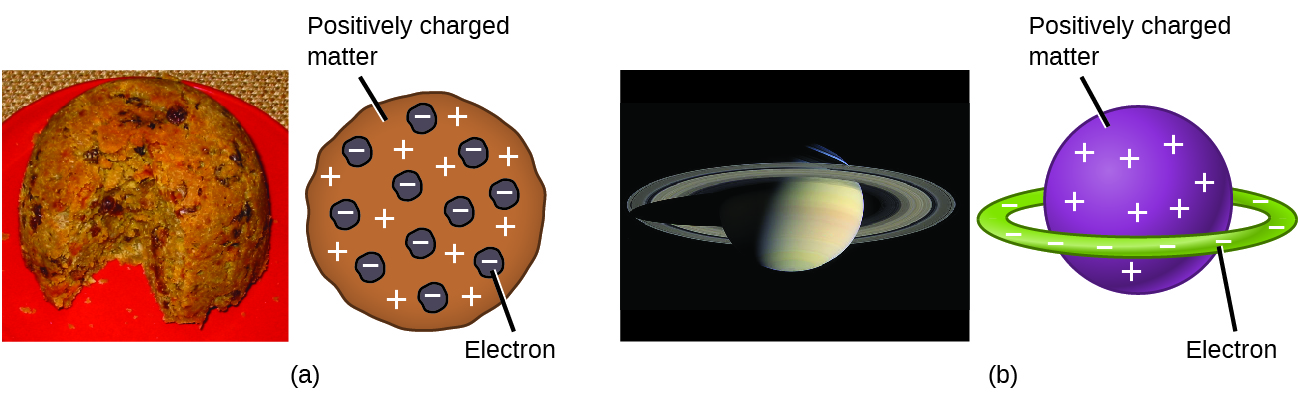
By the early 1900s, scientists had some understanding of the charge and mass of the negatively charged subatomic particle—the electrons. They also knew that atoms are electrically neutral, so there must be positively charged components in an atom. In 1904, an English physicist named J. J. Thomson proposed the “plum pudding” model of atoms, which described a positively charged mass with an equal amount of negative charge in the form of electrons embedded in it. A competing model had been proposed in 1903 by Hantaro Nagaoka, who postulated a Saturn-like atom, consisting of a positively charged sphere surrounded by a halo of electrons (Figure 1).
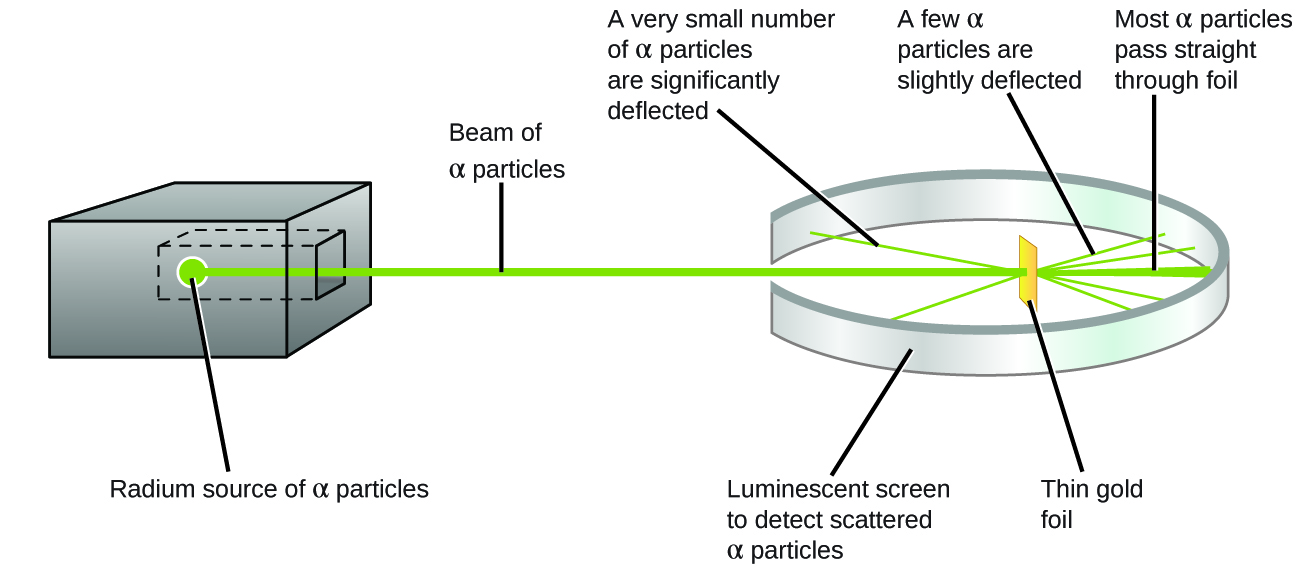
The next major development in understanding the atom came from Ernest Rutherford, a physicist from New Zealand who largely spent his scientific career in Canada and England. He performed a series of experiments using a beam of high-speed, positively charged alpha particles (α particles) that were produced by the radioactive decay of radium; α particles consist of two protons and two neutrons. Rutherford and his colleagues Hans Geiger (later famous for the Geiger counter) and Ernest Marsden aimed a beam of α particles, the source of which was embedded in a lead block to absorb most of the radiation, at a very thin piece of gold foil and examined the resultant scattering of the α particles using a luminescent screen that glowed briefly wherever hit by an α particle.
What did they discover? Most particles passed right through the foil without being deflected at all. However, some were diverted slightly, and a very small number (around 1 in 20,000) were deflected almost straight back toward the source (Figure 2). Rutherford later described finding these results: “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you”[1].
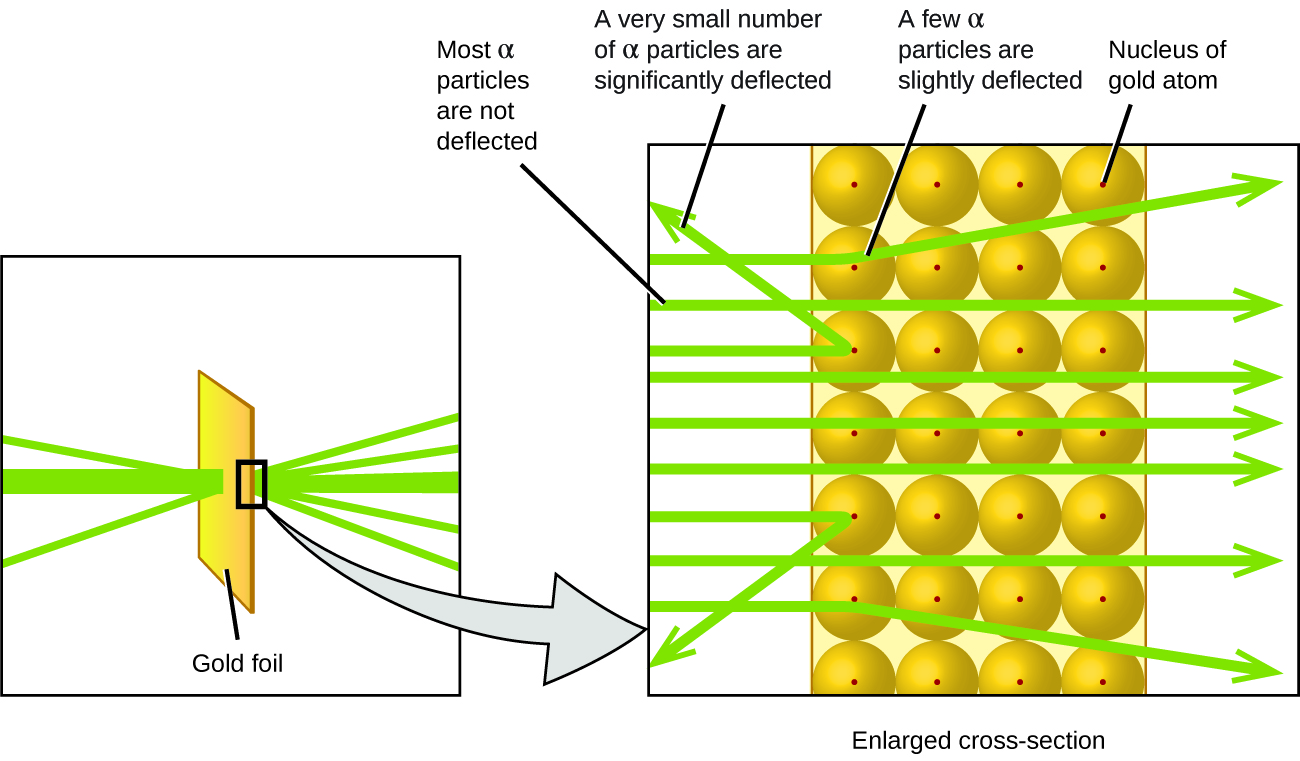
From these experimental results, Rutherford deduced that because most of the fast-moving α particles passed through the gold atoms undeflected, they must have traveled through essentially empty space inside the atom. Alpha particles are positively charged, so deflections arose when they encountered another positive charge (like charges repel each other). Therefore, the few positively charged α particles that changed paths abruptly must have hit, or closely approached, another body that also had a highly-concentrated positive charge. Since the deflections occurred a tiny fraction of the time, this charge only occupied a small amount of the space in the gold foil. Analyzing a series of such experiments in detail, Rutherford drew two conclusions:
- The volume occupied by an atom must consist of a large amount of empty space.
- A small, relatively heavy, positively charged body, the nucleus, must be at the center of each atom.

View this simulation of the Rutherford gold foil experiment. Adjust the slit width to produce a narrower or broader beam of α particles to see how that affects the scattering pattern.
This analysis led Rutherford to propose a model in which an atom consists of a very small, positively charged nucleus, in which most of the mass of the atom is concentrated. This nucleus is surrounded by the negatively charged electrons, such that the atom is electrically neutral (Figure 3). After many more experiments, Rutherford also discovered that the nuclei of other elements contain the hydrogen nucleus as a “building block,” and he named this more fundamental particle the proton, the positively charged, subatomic particle found in the nucleus. With one addition, which you will learn next, this basic nuclear model of the atom, proposed over a century ago, is still used today.

The Rutherford Scattering simulation allows you to investigate the differences between a “plum pudding” atom and a Rutherford atom by firing α particles at each type of atom.
Key Concepts and Summary
Rutherford’s famous gold foil experiment was performed by aiming α particles at a sheet of gold foil and observing whether the α particles passed through, deflected slightly, or deflected significantly. Because most of the α particles passed through undeflected, Rutherford deduced that the atom was mostly empty space. The deflection of some α particles indicated that there was a small region of positive charge that was repelling the positively charged α particles. This positively charged region must be the center of the atom.
Glossary
- alpha particles (α particles)
- positively charged particle consisting of two protons and two neutrons
- nucleus
- massive, positively charged center of an atom made of protons and neutrons
- proton
- positively charged, subatomic particle located in the nucleus
Chemistry End of Section Exercises
- Why did Rutherford and his team choose gold foil?
- Predict how Rutherford’s results would have been similar and how they’d be different if he’d used graphene instead of gold foil.
- Go back to the Rutherford Scattering PhET simulation you ran for your pre-class activity. Repeat the simulation, and this time use the smallest target (20 protons and 20 neutrons). How is the scattering affected by the calcium sheet, compared to the gold foil?
- Which of the following statements is false concerning Rutherford’s gold foil experiment?
- Alpha particles aimed at the gold foil traveled at high speed.
- Some alpha particles were reflected back towards the source.
- Gold was chosen because it can be pounded very thin.
- It was found that an atom is mostly empty space.
- Alpha particles were deflected once for every 100 particles.
Answers to Chemistry End of Section Exercises
- Gold foil is very malleable. It could be hammered to approximately 10 nm thick and remain strong.
- Like gold, graphene is also strong and malleable. The carbon atoms in graphene are smaller than gold atoms, and the carbon nuclei would have less protons and neutrons compared to gold atoms. Due to the lower mass of graphene and also due to the conservation of momentum, the collisions between alpha particles and graphene would be less elastic than collisions between alpha particles and gold. Taken all together, one would predict fewer back-scattered collisions with graphene, compared to with gold.
- There is less scattering in the calcium sheet than with the gold sheet. This is because a calcium nucleus is much smaller than a gold nucleus, so the likelihood of an alpha particle hitting a Ca nucleus is lower.
- E
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

