M14Q1: Introduction to Chemical Equilibrium; Qualitative View of Chemical Equilibrium; Disturbances to Equilibrium and System Responses; Le Châtelier’s Principle
Learning Objectives
- Recognize that all reactants and products will be present in a system at equilibrium, though not necessarily in equal amounts.
| Equilibrium | - Identify that once equilibrium is established, the rate of the forward reaction will be equal to the rate of the reverse reaction.
- Compare, for a system at equilibrium, the static nature of macroscopic observations with the dynamic nature of forward and reverse reactions at the molecular level.
- Interpret and construct qualitative graphs (concentration vs. time; reaction rate vs. time) for a given system as equilibrium is established.
- Use Le Châtelier’s Principle to predict how equilibrium is reestablished after a change in temperature, change in concentration or amount of a reactant or product, or change in volume or pressure (for a reaction involving gases).
| Le Châtelier’s Principle | - Utilize Le Châtelier’s principle to graph concentration vs. time qualitatively, showing how a system at equilibrium adjusts when disturbed by adding/removing reactants or products.
| Key Concepts and Summary | Glossary | End of Section Exercises |
Introduction
Imagine a beach populated with sunbathers and swimmers. As those basking in the sun get too hot and want to cool off, they head into the surf to swim. As the swimmers tire, they head to the beach to rest. If these two rates of transfer (sunbathers entering the water, swimmers leaving the water) are equal, the number of sunbathers and swimmers would be constant, or at equilibrium, although the identities of the people are constantly changing from sunbather to swimmer and back. An analogous situation occurs in chemical reactions. Reactions can occur in both directions simultaneously (reactants to products and products to reactants) and eventually reach a state of balance.
In this section, you will learn how to predict the position of the balance, the yield of a product of a reaction under specific conditions, how to change a reaction’s conditions to increase or reduce yield, and how to evaluate an equilibrium system’s reaction to disturbances.
Equilibrium
A chemical reaction is usually written in a way that suggests it proceeds in one direction, from left to right. But all chemical reactions are reversible and both the forward and reverse reaction occur to one degree or another depending on the reaction conditions. In a chemical equilibrium, the forward and reverse reactions occur at equal rates, and the concentrations of products and reactants remain constant, although not necessarily equal to each other. If we run a reaction in a closed system so that the products cannot escape, we often find the reaction does not yield 100% products. Instead, some reactants remain after the concentrations stop changing. At this point, when there is no further change in concentrations of reactants and products, we say the reaction is at equilibrium.
For example, when we place a sample of dinitrogen tetroxide (N2O4, a colorless gas) in a glass tube, it forms nitrogen dioxide (NO2, a brown gas) by the reaction:
N2O4(g) ⇌ 2 NO2(g)
The color becomes darker as N2O4 is converted to NO2. When the system reaches equilibrium, both N2O4 and NO2 are present (Figure 1). On the left side of the figure, there is only colorless N2O4 in the system. But as the reaction progresses and equilibrium is achieved (moving from left to right), we can observe the formation of brown NO2.
![There are two graphs. The left graph has a y-axis labeled, “Concentration,” and an x-axis labeled, “Time.” A red line labeled, “N O subscript 2,” begins in the bottom left corner of the graph at a point labeled, “0,” and rises near the highest point on the y-axis before it levels off and becomes horizontal. A blue line labeled, “N subscript 2 O subscript 4,” begins near the highest point on the y-axis and drops below the midpoint of the y-axis before leveling off. The right graph has a y-axis labeled, “Rate,” and an x-axis labeled, “Time.” A red line labeled, “k subscript f, [ N subscript 2 O subscript 4 ],” begins in the bottom left corner of the graph at a point labeled, “0,” and rises near the middle of the y-axis before it levels off and becomes horizontal. A blue line labeled, “k subscript f, [ N O subscript 2 ] superscript 2,” begins near the highest point on the y-axis and drops to the same point on the y-axis as the red line before leveling off. The point where both lines become horizontal is labeled, “Equilibrium achieved.”](https://wisc.pb.unizin.org/app/uploads/sites/557/2020/10/104_M3Q1_ratesequilibriumgraphcropped.png)
The formation of NO2 from N2O4 is a reversible reaction, which is identified by the equilibrium arrow (⇌). In a reversible reaction, the reactants can combine to form products and the products can combine to form reactants. Thus, not only can N2O4 decompose to form NO2, but the NO2 produced can react to form N2O4. As soon as the forward reaction produces any NO2, the reverse reaction begins and NO2 starts to react to form N2O4. At equilibrium, the concentrations of N2O4 and NO2 no longer change because the rate of NO2 formation is exactly equal to the rate of NO2 consumption, and the rate of formation of N2O4 is exactly equal to the rate of consumption of N2O4. Chemical equilibrium is a dynamic process: the numbers of reactant and product molecules remain constant, but there is a flux back and forth between them.
We can detect a state of equilibrium because the concentrations of reactants and products do not appear to change. However, it is important that we verify that the absence of change is due to equilibrium and not to a reaction rate that is so slow that changes in concentration are difficult to detect.
We use an equilibrium arrow when writing an equation for a reversible reaction. Such a reaction may or may not be at equilibrium. When we wish to speak about one particular component of a reversible reaction, we use a single arrow. For example, in the equilibrium shown in Figure 1, the rate of the forward reaction:
2 NO2(g) → N2O4(g)
is equal to the rate of the reverse reaction:
N2O4(g) → 2 NO2(g)
Chemistry in Real Life: Equilibrium and Soft Drinks
The connection between chemistry and carbonated soft drinks goes back to 1767, when Joseph Priestley (1733–1804; mostly known today for his role in the discovery and identification of oxygen) discovered a method of infusing water with carbon dioxide to make carbonated water. In 1772, Priestly published a paper entitled “Impregnating Water with Fixed Air.” The paper describes dripping oil of vitriol (today we call this sulfuric acid, but what a great way to describe sulfuric acid: “oil of vitriol” literally means “liquid nastiness”) onto chalk (calcium carbonate). The resulting CO2 falls into the container of water beneath the vessel in which the initial reaction takes place; agitation helps the gaseous CO2 mix into the liquid water.
H2SO4(ℓ) + CaCO3(s) → CO2(g) + H2O(ℓ) + CaSO4(aq)
Carbon dioxide is slightly soluble in water. There is an equilibrium reaction that occurs as the carbon dioxide reacts with the water to form carbonic acid (H2CO3). Since carbonic acid is a weak acid, it can dissociate into protons (H+) and hydrogen carbonate ions (HCO3–).
CO2(g) + H2O(ℓ) ⇌ H2CO3(aq) ⇌ HCO3–(aq) + H+(aq)
Today, CO2 can be pressurized into soft drinks, establishing the equilibrium shown above under a higher pressure condition. Once you open the beverage container, however, a cascade of equilibrium shifts occurs. First, the CO2 gas in the air space on top of the bottle escapes, causing the equilibrium between gas-phase CO2 and dissolved or aqueous CO2 to shift, lowering the concentration of CO2 in the soft drink. Less CO2 dissolved in the liquid leads to carbonic acid decomposing to dissolved CO2 and H2O. The lowered carbonic acid concentration causes a shift of the final equilibrium. The net result is that the CO2 bubbles up out of the beverage, releasing the gas into the air (Figure 2), results in a soft drink with a much lowered CO2 concentration, often referred to as “flat.”

Let us consider the evaporation of bromine as a second example of a system at equilibrium.
Br2(ℓ) ⇌ Br2(g)
An equilibrium can be established for a physical change—like this liquid to gas transition—as well as for a chemical reaction. Figure 3 shows a sample of liquid bromine at equilibrium with bromine vapor in a closed container. When we pour liquid bromine into an empty bottle in which there is no bromine vapor, some liquid evaporates: the amount of liquid decreases and the amount of vapor increases. If we cap the bottle so no vapor escapes, the amount of liquid and vapor will eventually stop changing and an equilibrium between the liquid and the vapor will be established. If the bottle were not capped, the bromine vapor would escape and no equilibrium would be reached.

Le Châtelier’s Principle
If a system at equilibrium is subjected to a perturbation or stress (such as a change in concentration) the position of equilibrium changes. This process is described by Le Châtelier’s principle: When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance.
Effect of Change in Concentration on Equilibrium
A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or products. The concentrations of both reactants and products then undergo additional changes to return the system to equilibrium.
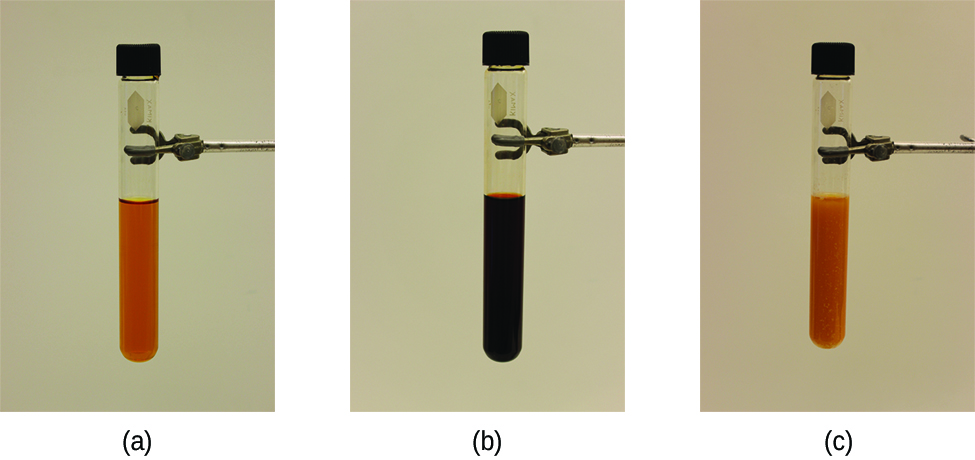
Consider Figure 4, which shows the progression of an equilibrium after two applied stresses. Originally, the test tube contains a solution of [Fe(H2O)6]3+ in water (left picture). Next thiocyanate, SCN–, is added to the test tube, which can react with [Fe(H2O)6]3+ to form a complex with iron, [Fe(SCN)(H2O)6]2+, resulting in an equilibrium between colorless SCN–, orange [Fe(H2O)6]3+, and dark red [Fe(SCN)(H2O)6]2+ (middle photo):
[Fe(H2O)6]3+(aq) + SCN−(aq) ⇌ [Fe(SCN)(H2O)6]2+(aq)
Then, silver ions, Ag+, are added to the test tube, which removes SCN– precipitates as a white solid, AgSCN(s) (right photo). The stress on the system in Figure 4 is the reduction of the equilibrium concentration of SCN−. Le Châtelier’s principle leads us to predict that the concentration of SCN– should increase, also increasing the concentration of [Fe(H2O)6]3+ and decreasing the concentration of [Fe(SCN)(H2O)6]2+ to restore an equilibrium relationship between all three.
Demonstration: Le Chatelier’s Principle
Set up. A solution of CoCl2(aq) is mixed with 12 M HCl solution, diluted with H2O, and then mixed with more HCl. When CoCl2 is added to water, a hydrated complex is formed:
CoCl2(aq) + 6 H2O(ℓ) ⇌ [Co(H2O)6]2+(aq) + 2 Cl–(aq)
Prediction. Write the equilibrium reaction needed to justify the changes when HCl(aq) and H2O are added to the [Co(H2O)6]2+solution. Predict what will happen when HCl is added, and then after H2O is added to dilute the solution.
Explanation: When CoCl2(aq) is dissolved, the pink, hydrated [Co(H2O)6]2+(aq) complex is formed. An equilibrium is established:
[Co(H2O)6]2+(aq) + 4 Cl–(aq) ⇌ [CoCl4]2-(aq) + 6 H2O(ℓ)
When 12 M HCl is added, additional Cl–(aq) ions are added to the equilibrium and the reaction shifts to the right, towards the products. As a result, the pink [Co(H2O)6]2+ complex is converted into the deep blue [CoCl4]2- complex.
When H2O is added, all the aqueous species are diluted and all of the concentrations decrease. The equilibrium will shift to increase all aqueous concentrations. There are 5 moles of aqueous species in the reactants and one mole in the products. The equilibrium shifts to the left, towards the reactants, since there are more aqueous species in the reactants.
When additional 12 M HCl is added, the equilibrium is flooded with chloride ions and once again shifts to the right, towards the products.
Effect of Change in Pressure on Equilibrium
Sometimes we can change the position of equilibrium by changing the pressure of a system. However, changes in pressure have a measurable effect only in systems in which gases are involved, and then only when the chemical reaction produces a change in the total number of gas molecules in the system. An easy way to recognize such a system is to look if the reactant and product sides of the equilibrium have a differing number of moles of gas. Some changes to total pressure, like adding an inert gas that is not part of the equilibrium, will change the total pressure but not the partial pressures of the gases in the equilibrium constant expression. Thus, addition of a gas not involved in the equilibrium will not perturb the equilibrium.

Check out this link to see a dramatic visual demonstration of how equilibrium changes with shifts in pressure.
As we increase the pressure of a gaseous system at equilibrium, either by decreasing the volume of the system or by adding more of one of the components of the equilibrium mixture, we introduce a stress by increasing the partial pressures of one or more of the components. In accordance with Le Châtelier’s principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored because this relieves the stress. The reverse reaction would be favored by a decrease in pressure.
Consider what happens when we increase the pressure (or decrease the container volume) on a system in which NO, O2, and NO2 are at equilibrium:
2 NO(g) + O2(g) ⇌ 2 NO2(g)
The formation of additional amounts of NO2 decreases the total number of gas molecules in the system because each time two molecules of NO2 form, a total of three molecules of NO and O2 are consumed. This reduces the total pressure exerted by the system and reduces, but does not completely relieve the stress of the increased pressure. On the other hand, a decrease in the pressure on the system favors decomposition of NO2 into NO and O2, which tends to restore the pressure.
Now consider this reaction:
N2(g) + O2(g) ⇌ 2 NO(g)
Because there is no change in the total number of gas molecules in the system during reaction, a change in pressure (or container volume) does not favor either formation or decomposition of gaseous nitrogen monoxide.
Effect of Change in Temperature on Equilibrium
Changing the temperature of a system at equilibrium has a different effect than those we have discussed so far. A change in temperature actually changes the value of the equilibrium constant. However, we can qualitatively predict the effect of the temperature change by treating it as a stress on the system and applying Le Châtelier’s principle.
When hydrogen reacts with gaseous iodine, heat is released.
H2(g) + I2(g) ⇌ 2 HI(g) ΔH = -9.4 kJ/mol (exothermic)
Because this reaction is exothermic, we can write it with heat as a product.
H2(g) + I2(g) ⇌ 2 HI(g) + heat
Increasing the temperature of the reaction increases the internal energy of the system. Thus, increasing the temperature has the effect of increasing the amount of one of the products of this reaction. The reaction shifts to the left to relieve the stress, and there is an increase in the concentration of H2 and I2 and a reduction in the concentration of HI. Lowering the temperature of this system reduces the amount of energy present, favors the production of heat, and favors the formation of hydrogen iodide.
Temperature affects the equilibrium between NO2 and N2O4 in this reaction
N2O4(g) ⇌ 2 NO2(g) ΔH = 57.20 kJ/mol
The positive ΔH value tells us that the reaction is endothermic and could be written
heat + N2O4(g) ⇌ 2 NO2(g)
At higher temperatures, the gas mixture has a deep brown color, indicative of a significant amount of brown NO2 molecules. If, however, we put a stress on the system by cooling the mixture (withdrawing energy), the equilibrium shifts to the left to supply some of the energy lost by cooling. The concentration of colorless N2O4 increases, and the concentration of brown NO2 decreases, causing the brown color to fade.
Demonstration: Le Chatelier’s Principle
Set up. Three flasks are filled with NO2(g) and N2O4(g). One flask is left at room temperature, one is heated in a hot water bath, and one is cooled in a ice bath.
Prediction. Write the equilibrium reaction between NO2(g) and N2O4(g). Predict whether the reaction is endothermic or exothermic.
Explanation: The equilibrium reaction within the flask are:
2 NO2(g) ⇌ N2O4(g)
When the reaction is heated, the reaction shifts to the red-brown NO2, towards the left. When the reaction is cooled, the reaction shifts to the colorless N2O4, towards the right.
When heat is added, the equilibrium shifts away from the heat, which in this case is to the left. When heat is removed, the equilibrium shifts towards the heat, which is to the right. Heat must be on the products side, and the reaction overall is exothermic.

This interactive animation allows you to apply Le Châtelier’s principle to predict the effects of changes in concentration, pressure, and temperature on reactant and product concentrations.
Catalysts Do Not Affect Equilibrium
As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and reverse reactions), a catalyst has no effect on the equilibrium concentrations.
Chemistry in Real Life: Ammonia
The interplay of changes in concentration or pressure, temperature, and the lack of an influence of a catalyst on a chemical equilibrium is illustrated in the industrial synthesis of ammonia from nitrogen and hydrogen according to the equation
N2(g) + 3 H2(g) ⇌ 2 NH3(g)
A large quantity of ammonia is manufactured by this reaction. Each year, ammonia is among the top 10 chemicals, by mass, manufactured in the world. About 2 billion pounds are manufactured in the United States each year.
Ammonia plays a vital role in our global economy. It is used in the production of fertilizers and is, itself, an important fertilizer for the growth of corn, cotton, and other crops. Large quantities of ammonia are converted to nitric acid, which plays an important role in the production of fertilizers, explosives, plastics, dyes, and fibers, and in the steel industry.
It has long been known that nitrogen and hydrogen react to form ammonia. However, it became possible to manufacture ammonia in useful quantities by the reaction of nitrogen and hydrogen only in the early 20th century after the factors that influence its equilibrium were understood.
To be practical, an industrial process must give a large yield of product relatively quickly. One way to increase the yield of ammonia is to increase the pressure on the system in which N2, H2, and NH3 are at equilibrium or are coming to equilibrium.
N2(g) + 3 H2(g) ⇌ 2 NH3(g)
The formation of additional amounts of ammonia reduces the total pressure exerted by the system and somewhat reduces the stress of the increased pressure.
Although increasing the pressure of a mixture of N2, H2, and NH3 will increase the yield of ammonia, at low temperatures, the rate of formation of ammonia is slow. At room temperature, for example, the reaction is so slow that if we prepared a mixture of N2 and H2, no detectable amount of ammonia would form during our lifetime. The formation of ammonia from hydrogen and nitrogen is an exothermic process:
N2(g) + 3 H2(g) ⇌ 2 NH3(g) ΔH = -92.2 kJ/mol
Thus, increasing the temperature to increase the rate lowers the yield. If we lower the temperature to shift the equilibrium to favor the formation of more ammonia, equilibrium is reached more slowly because of the large decrease of reaction rate with decreasing temperature.
The decrease in the rate of formation caused by operating at lower temperatures can be regained by using a catalyst. The net effect of the catalyst on the reaction is to cause equilibrium to be reached more rapidly.
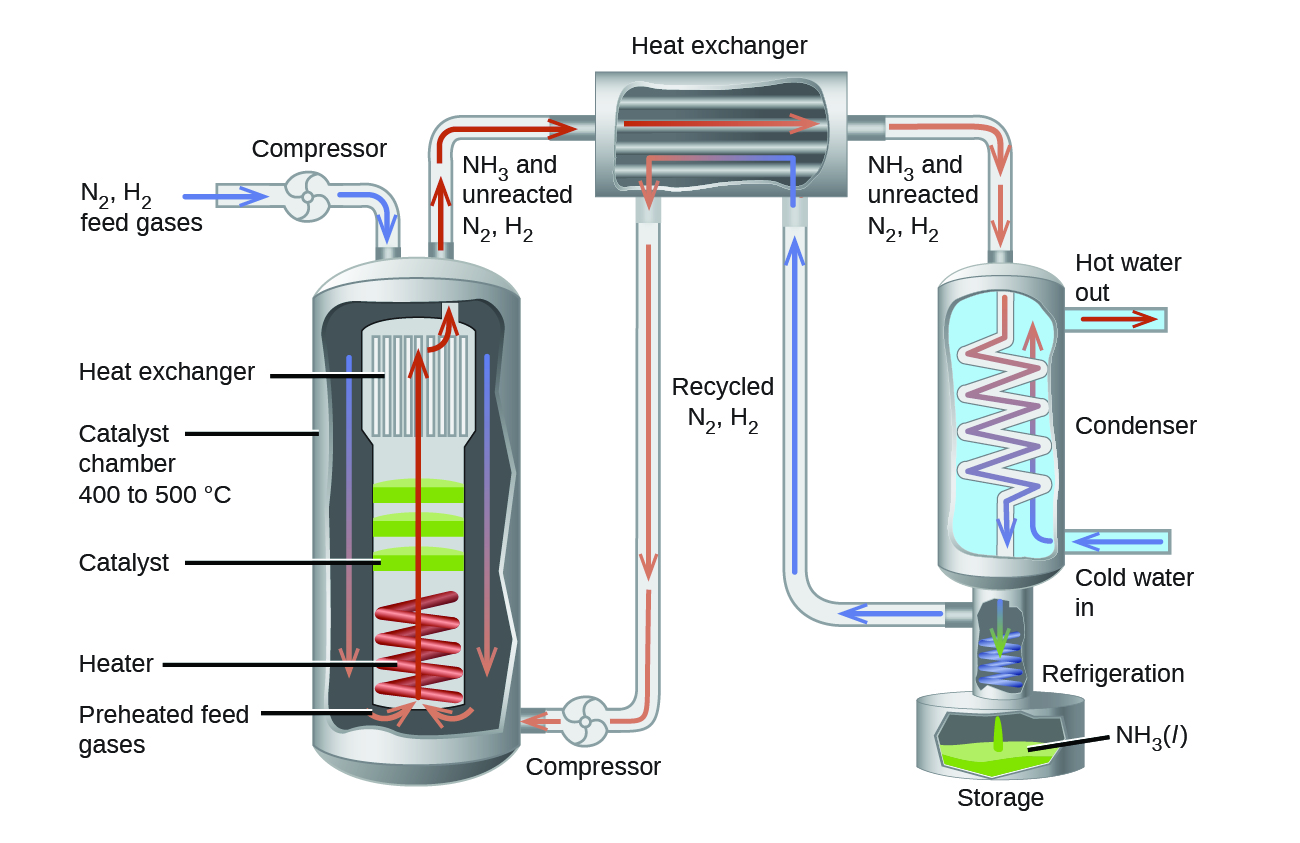
In the commercial production of ammonia, conditions of about 500 °C, 150–900 atm, and the presence of a catalyst are used to give the best compromise among rate, yield, and the cost of the equipment necessary to produce and contain high-pressure gases at high temperatures (Figure 5).
Chemistry in Real Life: Fritz Haber
In the early 20th century, German chemist Fritz Haber (Figure 6) developed a practical process for converting diatomic nitrogen, which cannot be used by plants as a nutrient, to ammonia, a form of nitrogen that is easiest for plants to absorb.
N2(g) + 3 H2(g) ⇌ 2 NH3(g)
The availability of nitrogen is a strong limiting factor to the growth of plants. Despite accounting for 78% of air, diatomic nitrogen (N2) is nutritionally unavailable due the tremendous stability of the nitrogen-nitrogen triple bond. For plants to use atmospheric nitrogen, the nitrogen must be converted to a more bioavailable form (this conversion is called nitrogen fixation).
Haber was born in Breslau, Prussia (presently Wroclaw, Poland) in December 1868. He went on to study chemistry and, while at the University of Karlsruhe, he developed what would later be known as the Haber process: the catalytic formation of ammonia from hydrogen and atmospheric nitrogen under high temperatures and pressures. For this work, Haber was awarded the 1918 Nobel Prize in Chemistry for synthesis of ammonia from its elements. The Haber process was a boon to agriculture, as it allowed the production of fertilizers to no longer be dependent on mined feed stocks such as sodium nitrate. Currently, the annual production of synthetic nitrogen fertilizers exceeds 100 million tons and synthetic fertilizer production has increased the number of humans that arable land can support from 1.9 persons per hectare in 1908 to 4.3 in 2008.

In addition to his work in ammonia production, Haber is also remembered by history as one of the fathers of chemical warfare. During World War I, he played a major role in the development of poisonous gases used for trench warfare. Regarding his role in these developments, Haber said, “During peace time a scientist belongs to the World, but during war time he belongs to his country.”[1] Haber defended the use of gas warfare against accusations that it was inhumane, saying that death was death, by whatever means it was inflicted. He stands as an example of the ethical dilemmas that face scientists in times of war and the double-edged nature of the sword of science.
Like Haber, the products made from ammonia can be multifaceted. In addition to their value for agriculture, nitrogen compounds can also be used to achieve destructive ends. Ammonium nitrate has also been used in explosives, including improvised explosive devices. Ammonium nitrate was one of the components of the bomb used in the attack on the Alfred P. Murrah Federal Building in downtown Oklahoma City on April 19, 1995.
Key Concepts and Summary
We have often treated chemical reactions as proceeding in one direction, from left to right. But in reality, chemical reactions are reversible and both the forward and reverse reactions can occur (although not necessarily equally!). When a chemical system reaches the point where the forward and reverse reactions occur at equal rates, we refer to this as being at chemical equilibrium. A system will remain at equilibrium unless a stress is applied. The way a system counteracts the stress and returns to equilibrium is dictated by Le Châtelier’s principle.
- Concentration: adding or removing gas or aqueous phase species will cause a shift in the equilibrium either away or towards the species. A species added not present in the chemical system can also cause a shift in the equilibrium if it reacts with a molecule in the chemical system.
- Pressure: Changing the pressure will potentially have an effect on reactions with gas phase species. Increasing the pressure causes a shift towards the side with fewer moles of gas phase species, and vice versa. Decreasing the volume of the container has the same effect as increasing the pressure.
- Volume of solution: Adding water (or diluting) a solution will potentially have an effect on reactions with aqueous phase species. Increasing the volume of water causes a shift towards the side with more moles of aqueous phase species, and vice versa.
- Temperature: Changing the temperature causes a shift depending on the enthalpy of the reaction. If the reaction is exothermic (-ΔH), increasing the temperature causes a shift towards reactants and vice versa. If the reaction is endothermic (+ΔH), increasing the temperature causes a shift towards the products and vice versa. Temperature is the only stress that changes the magnitude of the equilibrium constant.
- Catalyst: A catalyst does not cause a shift in the equilibrium.
Glossary
- equilibrium
- the point in a chemical reaction where the forward and reverse reactions occur at equal rates and the concentrations of products and reactants remain constant (although not necessarily equal)
- Le Châtelier’s principle
- when a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance
- reversible reaction
- the reactants can combine to form products and the products can combine to form reactants, symbolized by equilibrium arrows, ⇌
- stress
- a perturbation applied to a system at equilibrium
Chemistry End of Section Exercises
- What does it mean to describe a reaction as “reversible”?
- When writing an equation, how is a reversible reaction distinguished from a nonreversible reaction?
- If a reaction is reversible, when can it be said to have reached equilibrium?
- Is a system at equilibrium if the rate constants of the forward and reverse reactions are equal?
- If the concentrations of products and reactants are equal, is the system at equilibrium?
- The following equation represents a reversible decomposition:
CaCO3(s) ⇌ CaO(s) + CO2(g)
Under what conditions will decomposition proceed to completion so that no CaCO3 remains?
- Explain how to recognize the conditions under which changes in pressure would affect systems at equilibrium.
- What property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?
- The following reaction occurs when a burner on a gas stove is lit:
CH4(g) + 2 O2(g) ⇌ CO2(g) + 2 H2O(g)
Is an equilibrium among CH4, O2, CO2, and H2O established under these conditions? Explain your answer.
- Suggest four ways in which the concentration of hydrazine, N2H4, could be increased in an equilibrium described by the following equation:
N2(g) + 2 H2(g) ⇌ N2H4(g) ΔH = 95 kJ/mol
- Suggest four ways in which the concentration of PH3 could be increased in an equilibrium described by the following equation:
P4(g) + 6 H2(g) ⇌ 4 PH3(g) ΔH = 110.5 kJ/mol
- How will an increase in temperature affect each of the following equilibria? How will a decrease in the volume of the reaction vessel affect each?
- 2 NH3(g) ⇌ N2(g) + 3 H2(g) ΔH = 92 kJ/mol
- N2(g) + O2(g) ⇌ 2 NO(g) ΔH = 181 kJ/mol
- 2 O3(g) ⇌ 3 O2(g) ΔH = -224 kJ/mol
- CaO(s) + CO2(g) ⇌ CaCO3(s) ΔH = -176 kJ/mol
- How will an increase in temperature affect each of the following equilibria? How will a decrease in the volume of the reaction vessel affect each?
- 2 H2O(g) ⇌ 2 H2(g) + O2(g) ΔH = 484 kJ/mol
- N2(g) + 3 H2(g) ⇌ 2 NH3(g) ΔH = -92 kJ/mol
- 2 Br(g) ⇌ Br2(g) ΔH = -224 kJ/mol
- H2(g) + I2(g) ⇌ 2 HI(g) ΔH = 53 kJ/mol
- Methanol, a liquid fuel that could possibly replace gasoline, can be prepared from carbon monoxide and hydrogen at high temperature and pressure in the presence of a suitable catalyst. The enthalpy of reaction for this process is -90.2 kJ/mol.
- Write the equilibrium equation for this process.
- Write will happen to the concentrations of H2, CO, and CH3OH at equilibrium if more H2 is added?
- What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if CO is removed?
- What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if CH3OH is added?
- What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if the temperature of the system is increased?
- What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if more catalyst is added?
- Nitrogen and oxygen react at high temperatures. The enthalpy of reaction for this process is 181 kJ/mol.
- Write the equilibrium equation for this process.
- What will happen to the concentrations of N2, O2, and NO at equilibrium if more O2 is added?
- What will happen to the concentrations of N2, O2, and NO at equilibrium if N2 is removed?
- What will happen to the concentrations of N2, O2, and NO at equilibrium if NO is added?
- What will happen to the concentrations of N2, O2, and NO at equilibrium if the pressure on the system is increased by reducing the volume of the reaction vessel?
- What will happen to the concentrations of N2, O2, and NO at equilibrium if a catalyst is added?
- Ammonia is a weak base that reacts with water according to this equation:
NH3(g) + H2O(l) ⇌ NH4+(aq) + OH–(aq)
Will any of the following increase the percent of ammonia that is converted to the ammonium ion in water? Select all that apply. Explain briefly.
- Addition of NaOH.
- Addition of HCl.
- Addition of NH4Cl.
- Suggest two ways in which the equilibrium concentration of Ag+ can be reduced after solutions of AgNO3(aq) and NaCl(aq) are combined.:
AgNO3(aq) + NaCl(aq) ⇌ AgCl(s) + NaNO3(aq) ΔH = -65.9 kJ/mol
- Additional solid silver sulfate, a slightly soluble solid, is added to a solution of silver ion and sulfate ion at equilibrium with solid silver sulfate.
2 Ag+(aq) + SO42-(aq) ⇌ Ag2SO4(s)
Which of the following will occur? Select all that apply. Explain your answer briefly.
- Ag+ and SO42- concentrations will not change.
- Additional silver sulfate will form and precipitate.
- The Ag+ concentration will increase and the SO42- concentration will decrease.
- NH4CO2NH2(s) is placed in a sealed flask and equilibrium is established. Which of the following disturbances to the system would cause the formation of more CO2, as equilibrium is reestablished? Select all that apply.
NH4CO2NH2(s) ⇌ 2 NH3(g) + CO2(g)
- Addition of more NH4CO2NH2(s).
- Removal of NH3(g).
- Increasing the volume of the reaction flask.
- Consider the chemical equilibrium shown below, which is carried out in a sealed container:
2 NOBr(g) ⇌ 2 NO(g) + Br2(g) ΔHrxn° = +30 kJ/mol
- Which change listed below will shift the position of the equilibrium to favor the formation of more products?
- Decrease the volume of the container
- Add more NO
- Remove Br2
- Lower the temperature
- Remove NOBr
- Which change listed below will cause the magnitude of the equilibrium constant to decrease?
- Lower the temperature
- Add some NO
- Remove some Br2
- Increase the volume of the container
- Add a catalyst
- Which change listed below will shift the position of the equilibrium to favor the formation of more products?
- The following is the storied Haber-Bosch process for mass-producing ammonia.
3 H2(g) + N2(g) ⇌ 2 NH3(g) ΔH = -92.2 kJ/mol
In order to make ammonia with optimal yield, which should you do? Select all that apply.
- Make sure no inert gas increases the pressure of the reaction vessel.
- Apply steady heat to the mixture.
- Keep the reaction container cool.
- Decrease the volume.
- Extract/trap ammonia as it is formed.
- Consider the following equilibrium. Which of these statements is correct? Select all that apply. (Note: the copper hexahydrate ion ([Cu(H2O)6]2+) is light blue and the copper ammine complex ([Cu(NH3)4(H2O)2]2+) is deep navy blue.)
[Cu(H2O)6]2+(aq) + 4 NH3(aq) ⇌ [Cu(NH3)4(H2O)2]2+(aq) + 4 H2O(ℓ)
- When the system is diluted, the system re-equilibrates by shifting right.
- If acid is added to react with ammonia, removing it from the above equilibrium, the reaction will respond by shifting to the left.
- Addition of potassium chloride, leading to the formation of CuCl42- from [Cu(H2O)6]2+ will cause a shift toward to right.
- When concentrated NH3 is added to [Cu(H2O)6]2+, the solution turns dark blue.
- Addition of water to the system does not cause the equilibrium to shift, despite the fact that water is a product.
Answers to Chemistry End of Section Exercises
- The reaction can proceed in both the forward and reverse directions.
- The use of bi-directional arrows in the reaction equation (⇌) denotes a reversible reaction. The use of a uni-directional arrow (→) denotes a non-reversible reaction.
- When a system has reached equilibrium, no further changes in the reactant and product concentrations occur. Both the forward and reverse reactions continue to occur, but at equivalent rates.
- No, the forward and reverse rates of the equilibrium system are equal but the forward and reverse rate constants are no necessarily equal to one another. In fact, it is most likely the forward and reverse rate constant will not be equal to one another.
- The concept of equilibrium does not imply equal concentrations, though it is possible.
- If the reaction occurs in an open-container, allowing the newly formed CO2 to escape, an equilibrium will not be established as the CO2 concentration cannot become constant.
- Changing pressure will affect equilibrium systems when:
- The reaction is taking placed in a closed system.
- At least one reactant or product is in a gas state.
- There are a different number of moles of gas particles on the reactant- and product-side of the reaction.
- The change in enthalpy (ΔH) may be used. If the reaction is exothermic, the heat produced, can be thought of as a product and if the reaction is endothermic, the heat added can be thought of as a reactant. Additional heat would shift an exothermic reaction back to the reactants but would shift an endothermic reaction to the products. Cooling an exothermic reaction causes the reaction to shift toward the product side; cooling an endothermic reaction would cause it to shift to the reactants’ side.
- No, it is not at equilibrium. Because the system is not confined, products continuously escape from the region of the flame; reactants are also added continuously from the burner and surrounding atmosphere.
- Some of the possibilities:
- Add N2. Increasing a reactant concentration shifts the equilibrium position toward the product-side of the reaction, resulting in an increased product concentration.
- Add H2. Increasing a reactant concentration shifts the equilibrium position toward the product-side of the reaction, resulting in an increased product concentration.
- Decrease the container volume. There are two moles of gas on the reactant-side of the reaction and only one mole of gas on the product-side. Decreasing the volume of the reaction system will increase the pressure of the system. The equilibrium will shift to reduce the pressure by moving to the reaction side with fewer gas moles.
- Heat the mixture. This is an endothermic reaction. If energy is then considered to be a reactant, addition of energy will result in a shift of the equilibrium position to the product-side of the reaction.
- Add P4. Add H2. Decrease the container volume. Heat the mixture.
- (a) ΔT increase = shift toward products, ΔP increase = shift toward reactants;
(b) ΔT increase = shift toward products, ΔP increase = no effect;
(c) ΔT increase = shift toward reactants, ΔP increase = shift toward reactants;
(d) ΔT increase = shift toward reactants, ΔP increase = shift toward products. - (a) ΔT increase = shift toward products, ΔP increase = shift toward reactants;
(b) ΔT increase = shift toward reactants, ΔP increase = shift toward products;
(c) ΔT increase = shift toward reactants, ΔP increase = shift toward products;
(d) ΔT increase = shift toward products, ΔP increase = no effect. - (a) 2 H2(g) + CO(g) ⇌ CH3OH(g) ΔH = -90.2 kJ/mol;
(b) [H2] increases, [CO] decreases, [CH3OH] increases;
(c) [H2] increases, [CO] decreases, [CH3OH] decreases;
(d) [H2] increases, [CO] increases, [CH3OH] increases;
(e) [H2] increases, [CO] increases, [CH3OH] decreases;
(f) no changes. - (a) N2(g) + O2(g) ⇌ 2 NO(g) ΔH = 181 kJ/mol;
(b) [N2] decreases, [O2] increases, [NO] increases;
(c) [N2] decreases, [O2] increases, [NO] decreases;
(d) [N2] increases, [O2] increases, [NO] increases;
(e) no changes;
(f) no changes. - Only B. HCl will react with the OH–, decreasing the OH– concentration and shifting the equilibrium position toward the products.
- Add NaCl or some other salt that produces Cl− to the solution, shifting the equilibrium to the right. Cooling the solution shifts the equilibrium to the right.
- Only A. Adding the solid silver sulfate to an already established equilibrium will have no effect. It will just sink to the bottom of the beaker.
- B and C
- (a) C; (b) A
- C, D, and E
- B and D
Left-click here to watch Exercise 22 problem solving video.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂
- Herrlich, P. “The Responsibility of the Scientist: What Can History Teach Us About How Scientists Should Handle Research That Has the Potential to Create Harm?” EMBO Reports 14 (2013): 759–764. ↵

