M3Q5-6: Oxidation-Reduction Reactions
Introduction
This section continues to explore aqueous chemical reactions. First, we examine what happens during oxidation-reduction reactions. Second, we analyze oxidation-reduction reactions to identify which reactant was oxidized, which reactant was reduced, the oxidizing agent, and the reducing agent. The section below provides a more detailed description of these topics, worked examples, practice problems and a glossary of important terms.
Learning Objectives for Oxidation-Reduction Reactions
- Assign oxidation numbers for elements in a species; identify which element is oxidized (or reduced) in a chemical reaction; identify the oxidizing and reducing agents.
| Oxidation-Reduction Reactions | - Use the Activity Series to predict if a chemical reaction will occur.
| Activity Series |
| Key Concepts and Summary | Glossary | End of Section Exercises |
Oxidation-Reduction Reactions
Earth’s atmosphere contains about 20% molecular oxygen, O2, a chemically reactive gas that plays an essential role in the metabolism of aerobic organisms and in many environmental processes that shape the world. The term oxidation was originally used to describe chemical reactions involving O2, but its meaning has evolved to refer to a broad and important reaction class known as oxidation-reduction (redox) reactions. A few examples of such reactions will be used to develop a clear picture of this classification.
Some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium chloride:
2 Na(s) + Cl2(g) ⟶ 2 NaCl(s)
It is helpful to view the process with regard to each individual reactant in the form of an equation called a half-reaction:
Na(s) ⟶ Na+(s) + e–
Cl2(g) + 2 e– ⟶ 2 Cl–(s)
These equations show that Na atoms lose electrons while Cl atoms (in the Cl2 molecule) gain electrons, the “(s)” designation for the resulting ions signify that they are present in the form of a solid ionic compound. For redox reactions of this sort, the loss and gain of electrons define the complementary processes that occur:
oxidation: loss of electrons
reduction: gain of electrons
In this reaction, then, sodium is oxidized and chlorine undergoes reduction. Viewed from a more active perspective, sodium functions as a reducing agent (reductant), since it provides electrons to (or reduces) chlorine. Likewise, chlorine functions as an oxidizing agent (oxidant), as it effectively removes electrons from (oxidizes) sodium.
reducing agent: species that is oxidized
oxidizing agent: species that is reduced
Some redox processes, however, do not involve the transfer of electrons. Consider, for example, a reaction similar to the one yielding NaCl:
H2(g) + Cl2(g) ⟶ 2 HCl(g)
The product of this reaction is a covalent compound, so transfer of electrons in the explicit sense is not involved. To clarify the similarity of this reaction to the previous one and permit an unambiguous definition of redox reactions, a property called oxidation number has been defined. The oxidation number (or oxidation state) of an element in a compound is the charge its atoms would possess if the compound were ionic. The following guidelines are used to assign oxidation numbers to each element in a molecule or ion.
- The oxidation number of an atom in an elemental substance is zero.
- The oxidation number of a monatomic ion is equal to the ion’s charge.
- Oxidation numbers for common nonmetals are usually assigned as follows:
- Hydrogen: +1 when combined with nonmetals, −1 when combined with metals
- Oxygen: −2 in most compounds, sometimes −1 (so-called peroxides, O22−), very rarely -½ (so-called superoxides, O2−), positive values when combined with F (values vary)
- Halogens: −1 for F always, −1 for other halogens except when combined with oxygen or other halogens (positive oxidation numbers in these cases, varying values)
- The sum of oxidation numbers for all atoms in a molecule or polyatomic ion equals the charge on the molecule or ion.
Note: The proper convention for reporting charge is to write the number first, followed by the sign (e.g., 2+), while oxidation number is written with the reversed sequence, sign followed by number (e.g., +2). This convention aims to emphasize the distinction between these two related properties.
Example 1
Assigning Oxidation Numbers
Follow the guidelines in this section of the text to assign oxidation numbers to all the elements in the following species:
- H2S
- SO32−
- Na2SO4
Solution
(a) According to guideline 1, the oxidation number for H is +1.
Using this oxidation number and the compound’s formula, guideline 4 may then be used to calculate the oxidation number for sulfur:
charge on H2S = 0
0 = 2(+1) + 1x
x = -2
(b) Guideline 3 suggests the oxidation number for oxygen is -2.
Using this oxidation number and the ion’s formula, guideline 4 may then be used to calculate the oxidation number for sulfur:
charge on SO32- = -2
-2 = 3(-2) + 1x
-2 + 6 = 1x
x = +4
(c) For ionic compounds, it’s convenient to assign oxidation numbers for the cation and anion separately.
According to guideline 2, the oxidation number for sodium is +1.
Assuming the usual oxidation number for oxygen (-2 per guideline 3), the oxidation number for sulfur is calculated as directed by guideline 4:
charge on SO42- = -2
-2 = 4(-2) + 1x
-2 + 8 = 1x
x = +6
Check Your Learning
Assign oxidation states to the elements whose atoms are underlined in each of the following compounds or ions:
- KNO3
- AlH3
- NH4+
- H2PO4−
Answer:
(a) N, +5; (b) Al, +3; (c) N, −3; (d) P, +5
Using the oxidation number concept, an all-inclusive definition of redox reaction has been established. Oxidation-reduction (redox) reactions are those in which one or more elements involved undergo a change in oxidation number. (While the vast majority of redox reactions involve changes in oxidation number for two or more elements, a few interesting exceptions to this rule do exist Example 2.) Definitions for the complementary processes of this reaction class are correspondingly revised as shown here:
oxidation: increase in oxidation number
reduction: decrease in oxidation number
Returning to the reactions used to introduce this topic, they may now both be identified as redox processes. In the reaction between sodium and chlorine to yield sodium chloride, sodium is oxidized (its oxidation number increases from 0 in Na to +1 in NaCl) and chlorine is reduced (its oxidation number decreases from 0 in Cl2 to −1 in NaCl). In the reaction between molecular hydrogen and chlorine, hydrogen is oxidized (its oxidation number increases from 0 in H2 to +1 in HCl) and chlorine is reduced (its oxidation number decreases from 0 in Cl2 to −1 in HCl).
Several subclasses of redox reactions are recognized, including combustion reactions in which the reductant (also called a fuel) and oxidant (often, but not necessarily, molecular oxygen) react vigorously and produce significant amounts of heat, and often light, in the form of a flame. One example includes solid rocket-fuel reactions, which are combustion processes. A typical propellant reaction in which solid aluminum is oxidized by ammonium perchlorate is represented by this equation:
10 Al(s) + 6 NH4ClO4(s) ⟶ 4 Al2O3(s) + 2 AlCl3(s) + 12 H2O(g) + 3 N2(g)

Watch a brief video showing the test firing of a small-scale, prototype, hybrid rocket engine planned for use in the new Space Launch System being developed by NASA. The first engines firing at 3 seconds (green flame) use a liquid fuel/oxidant mixture, and the second, more powerful engines firing at 4 seconds (yellow flame) use a solid mixture.
Single-displacement (replacement) reactions are redox reactions in which an ion in solution is displaced (or replaced) via the oxidation of a metallic element. One common example of this type of reaction is the acid oxidation of certain metals:
Zn(s) + 2 HCl(aq) ⟶ ZnCl2(aq) + H2(g)
Metallic elements may also be oxidized by solutions of other metal salts; for example:
Cu(s) + 2 AgNO3(aq) ⟶ Cu(NO3)2(aq) + 2 Ag(s)
This reaction may be observed by placing copper wire in a solution containing a dissolved silver salt. Silver ions in solution are reduced to elemental silver at the surface of the copper wire, and the resulting Cu2+ ions dissolve in the solution to yield a characteristic blue color (Figure 1).
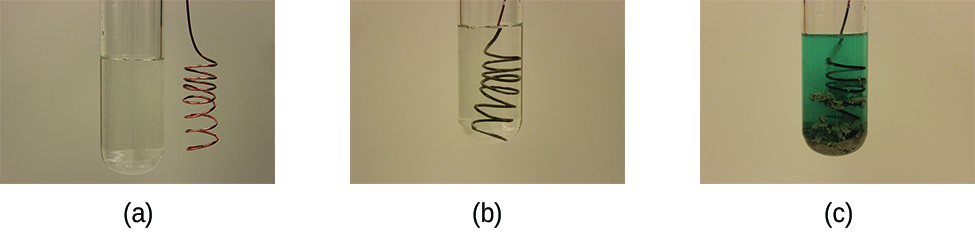
Example 2
Describing Redox Reactions
Identify which equations represent redox reactions, providing a name for the reaction if appropriate. For those reactions identified as redox, name the oxidant and reductant. Also, identify the oxidizing and reducing agents, as appropriate.
- ZnCO3(s) ⟶ ZnO(s) + CO2(g)
- Ga(ℓ) + 3 Br2(ℓ) ⟶ 2 GaBr3(s)
- 2 H2O2(aq) ⟶ 2 H2O(ℓ) + O2(g)
- BaCl2(aq) + K2SO4(aq) ⟶ BaSO4(s) + 2 KCl(aq)
- C2H4(g) + 3 O2(g) ⟶ 2 CO2(g) + 2 H2O(ℓ)
Solution
Redox reactions are identified per definition if one or more elements undergo a change in oxidation number.
- This is not a redox reaction, since oxidation numbers remain unchanged for all elements.
- This is a redox reaction. Gallium is oxidized, its oxidation number increasing from 0 in Ga(ℓ) to +3 in GaBr3(s). The reducing agent is Ga(ℓ). Bromine is reduced, its oxidation number decreasing from 0 in Br2(ℓ) to −1 in GaBr3(s). The oxidizing agent is Br2(ℓ).
- This is a redox reaction. It is a particularly interesting process, as it involves the same element, oxygen, undergoing both oxidation and reduction (a so-called disproportionation reaction). Oxygen is oxidized, its oxidation number increasing from −1 in H2O2(aq) to 0 in O2(g). Oxygen is also reduced, its oxidation number decreasing from −1 in H2O2(aq) to −2 in H2O(ℓ). For disproportionation reactions, the same substance functions as an oxidant and a reductant.
- This is not a redox reaction, since oxidation numbers remain unchanged for all elements.
- This is a redox reaction (combustion). Carbon is oxidized, its oxidation number increasing from −2 in C2H4(g) to +4 in CO2(g). The reducing agent (fuel) is C2H4(g). Oxygen is reduced, its oxidation number decreasing from 0 in O2(g) to −2 in H2O(ℓ). The oxidizing agent is O2(g).
Check Your Learning
This equation describes the production of tin(II) chloride:
Sn(s) + 2 HCl(g) ⟶ SnCl2(s) + H2(g)
Is this a redox reaction? If so, provide a more specific name for the reaction if appropriate, and identify the oxidant and reductant.
Answer:
Yes, a single-replacement reaction. Sn(s) is the reducing agent; HCl(g) is the oxidizing agent.
Activity Series
Single-replacement reactions only occur when the element that is doing the replacing is more reactive than the element that is being replaced. Therefore, it is useful to have a list of elements in order of their relative reactivities. The activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Table 1 is an activity series of most common metals.
| metal | description | |
| Easiest to oxidize; Strongest reducing agents |
Li K Ba Sr Ca Na |
React with cold water, replacing hydrogen. |
| Mg Al Zn Cr Fe Cd |
React with steam, but not cold water, replacing hydrogen. | |
| Co Ni Sn Pb |
Do not react with water. React with acids, replacing hydrogen. | |
| H2 | ||
| Most difficult to oxidize; Weakest reducing agents |
Cu Ag Hg Pt Au |
Unreactive with water or acids. |
For a single-replacement reaction, a given element is capable of replacing an element that is below it in the activity series. This can be used to predict if a reaction will occur. Suppose that small pieces of the metal nickel were placed into two separate aqueous solutions: one of iron(III) nitrate and one of lead(II) nitrate. Looking at the activity series, we see that nickel is below iron, but above lead. Therefore, the nickel metal will be capable of replacing the lead in a reaction, but will not be capable of replacing iron. Note that the nickel metal does not replace lead metal, but rather Pb2+(aq) ions from solution. Pb(s) is a product of the reaction.
Ni(s) + Pb(NO3)2(aq) → Ni(NO3)2(aq) + Pb(s)
Ni(s) + Fe(NO3)3(aq) → NR (no reaction)
Demonstration: The activity series predicts whether reactions occur
Set up. In the following demonstrations, we will compare Cu(s), Zn(s), and H2(g).
In the first video, a piece of solid copper is placed into a solution of zinc sulfate, and a piece of solid zinc is placed into a solution of copper sulfate.
Prediction. Start by writing out the reaction equations for these reactions, then predict which, if either, will occur.
Explanation. The reactions shown in the above video are:
Cu(s) + ZnSO4(aq) → NR
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
You can observe the reaction that occurred on the piece of zinc metal because when the zinc is removed from the solution, it is a different color as some copper has deposited on the surface. This happens because zinc is above copper on the activity series and is more easily oxidized.
Set up. In the next video, copper is heated and reacts with oxygen in the air to form black copper oxide, CuO. After a layer of CuO has formed on the copper metal, the flame is removed and hydrogen gas is reacted with the CuO.
Prediction. Start by writing the reaction equation for the reaction of CuO and hydrogen gas to predict whether a reaction will occur.
Explanation. The reaction in this demonstration is:
CuO(s) + H2(g) → Cu(s) + H2O(g)
Since hydrogen is above copper on the activity series, it is capable of displacing the copper in copper(II) oxide and reducing it to solid copper. This is visible with the color change as the solid transitions from black CuO to copper color when exposed to H2(g).
Notice that all of the elements in this activity series are metals except for hydrogen. Metals above hydrogen in this series react with acids and displace H+(aq) ions from solution to form hydrogen gas. The less reactive metals below hydrogen in the series will generally not react with acids, unless the acid contains an anion that is a powerful oxidizing agent such as nitric acid, HNO3. The alkali metals (Group 1) and selected alkaline earth metals (Group 2) are reactive enough to displace hydrogen from water. For example, lithium will react with cold water, replacing hydrogen. It will also react with steam and with acids, since that requires a lower degree of reactivity. The reaction of the alkali metals Li, Na, and K with water to yield hydrogen gas and the metal hydroxide is a distinctive reaction of the Group I metals, the vigor of the reaction increasing as you go down the group as shown in the following demonstration.
Demonstration: Alkali metals react vigorously with water
Set up. Alkali metals react readily with water to yield H2(g) and metal hydroxides. This demonstration shows Li, Na, and K reactions. Phenolphthalein is an indicator that is added to the water before the metal. This acid-base indicator is colorless in acidic or neutral conditions, but turns pink in basic conditions (with an excess of OH–).
Explanation. All three metals actually float as they are less dense than water, and the Na and K melt into liquid spheres upon contact with the water. You can see that gas is produced in these reactions because there are bubbles and a fizzing sound is audible. The pink color indicates that the solution has become basic as basic hydroxide ions are produced. The general equation for this single displacement reaction is outlined below:
2 M(s) + 2 H2O(ℓ) ⟶ 2 MOH(aq) + H2(g) M = Group 1 or Alkali Metal
Curious how the rubidium and cesium, the two alkali metals below potassium, would react? Watch the reaction with five of the alkali metals with Rb and Cs at the very end.
Example 3
Use the Activity Series to Predict Reactions
Use the activity series to predict if the following reactions will occur. If not, write NR. If the reaction does occur, write the products of the reaction and balance the equation.
Al(s) + Zn(NO3)2(aq) →
Solution
Step 1: Plan the problem.
For A, compare the placements of aluminum and zinc on the activity series. For B, compare the placements of silver and hydrogen.
Step 2: Solve.
Since aluminum is above zinc, it is capable of replacing it and a reaction will occur. The products of the reaction will be aqueous aluminum nitrate and solid zinc. Take care to write the correct formulas for the products before balancing the equation. Aluminum adopts a +3 charge in an ionic compound, so the formula for aluminum nitrate is Al(NO3)3. The balanced equation is:
2 Al(s) + 3 Zn(NO3)2(aq) → 2 Al(NO3)3(aq) + 3 Zn(s)
Check Your Learning
Use the activity series to predict if the following reactions will occur. If not, write NR. If the reaction does occur, write the products of the reaction and balance the equation.
Ag(s) + HCl(aq) →
Answer:
Since silver is below hydrogen, it is not capable of replacing hydrogen in a reaction with an acid.
Ag(s) + HCl(aq) → NR
Key Concepts and Summary
Chemical reactions are classified according to similar patterns of behavior. Redox reactions involve a change in oxidation number for one or more reactant elements. Single-displacement reactions are an example of oxidation-reduction reactions. The activity series is helpful to predict if a chemical reaction will occur (or not).
Glossary
- combustion reaction
- vigorous redox reaction producing significant amounts of energy in the form of heat and, sometimes, light
- oxidation-reduction reaction
- (also, redox reaction) reaction involving a change in oxidation number for one or more reactant elements
- oxidation number
- (also, oxidation state) the charge each atom of an element would have in a compound if the compound were ionic
- oxidizing agent
- (also, oxidant) substance that brings about the oxidation of another substance, and in the process becomes reduced
- reducing agent
- (also, reductant) substance that brings about the reduction of another substance, and in the process becomes oxidized
- single-displacement reaction
- (also, replacement) redox reaction involving the oxidation of an elemental substance by an ionic species
Chemistry End of Section Exercises
- Indicate what type, or types, of reaction each of the following represents:
- H2O(g) + C(s) ⟶ CO(g) + H2(g)
- 2 KClO3(s) ⟶ 2 KCl(s) + 3 O2(g)
- Al(OH)3(aq) + 3 HCl(aq) ⟶ AlCl3(aq) + 3 H2O(ℓ)
- Pb(NO3)2(aq) + H2SO4(aq) ⟶ PbSO4(s) + 2 HNO3(aq)
- Silver can be separated from gold because silver dissolves in nitric acid while gold does not. Is the dissolution of silver in nitric acid an acid-base reaction or an oxidation-reduction reaction? Explain your answer.
- Determine the oxidation states of each of the elements in the following compounds:
- NaI
- GdCl3
- LiNO3
- H2Se
- Mg2Si
- RbO2, rubidium superoxide
- HF
- Determine the oxidation states of the elements in the compounds listed. None of the oxygen-containing compounds are peroxides or superoxides.
- H2SO4
- Ca(OH)2
- BrOH
- ClNO2
- TiCl4
- NaH
- Classify the following as acid-base reactions or oxidation-reduction reactions:
- Na2S(aq) + 2 HCl(aq) ⟶ 2 NaCl(aq) + H2S(g)
- 2 Na(s) + 2 HCl(aq) ⟶ 2 NaCl(aq) + H2(g)
- Mg(s) + Cl2(g) ⟶ MgCl2(aq)
- MgO(s) + 2 HCl(aq) ⟶ MgCl2(s) + H2O(ℓ)
- K3P(s) + 2 O2(g) ⟶ K3PO4(s)
- KOH(aq) + H3PO4(aq) ⟶ K3PO4(aq) + 3 H2O(ℓ)
- In the following reaction, what is the reducing agent?
2 N2H4(g) + N2O4(g) → 3 N2(g) + 4 H2O(g)
- Identify the atoms that are oxidized and reduced and the oxidizing and reducing agents in each of the following equations:
- Mg(s) + NiCl2(aq) ⟶ MgCl2(aq) + Ni(s)
- PCl3(ℓ) + Cl2(g) ⟶ PCl5(s)
- Zn(s) + H2SO4(aq) ⟶ ZnSO4(aq) + H2(g)
- 3 Cu(s) + 8 HNO3(aq) ⟶ 3 Cu(NO3)2(aq) + 2 NO(g) + 4 H2O(ℓ)
- Complete and balance the following oxidation-reduction reactions which give the highest possible oxidation state for the oxidized atoms.
- Al(s) + F2(g) ⟶
- Al(s) + CuBr2(aq) ⟶
- P4(s) + O2(g) ⟶
- Complete and balance the following oxidation-reduction reactions, and state the oxidizing and reducing agents.
- K(s) + H2O(ℓ) ⟶
- Ba(s) + HBr(aq) ⟶
- Sn(s) + I2(s) ⟶
- True or False: Based on the activity series in Table 1, we can predict that BaCl2(aq) will react with Na(s).
- Use the activity series in Table 1 to determine if the following reactions will occur as written.
- Pt(s) + Cu(NO3)2(aq) → Pt(NO3)2(aq) + Cu(s)
- Sn(s) + Cu(NO3)2(aq) → Sn(NO3)2(aq) + Cu(s)
- Which of the following is false?
- The mass of an atom is due primarily to its nucleus.
- The volume of an atom is due primarily to its electrons.
- All isotopes of a given element contain the exact same number of protons.
- A molecule must consist of atoms of at least two different elements.
- The oxidation number of a monatomic ion is equal to its charge.
Answers to Chemistry End of Section Exercises
- (a) oxidation-reduction (gas-forming)
(b) oxidation-reduction (gas-forming)
(c) acid-base (neutralization)
(d) double-replacement reaction - This is a redox reaction because there is a change in silver’s oxidation number. Ag → Ag+ when dissolved in acid.
- (a) Na +1, I -1
(b) Gd +3. Cl -1
(c) Li +1, N +5, O -2
(d) H +1, Se -2
(e) Mg +2, Si -4
(f) Rb +1, O2 -1
(g) H +1, F -1 - (a) H +1, S +6, O -2
(b) Ca +2, O -2, H +1
(c) Br +1, O -2, H +1
(d) Cl -1, N +5, O -2
(e) Ti +4, Cl -1
(f) Na +1, H -1Left-click here to watch Exercise 4 problem solving video.
- (a) acid-base; (b) oxidation-reduction; (c) oxidation reduction;
(d) acid-base; (e) oxidation-reduction; (f) acid-base - N2H4
- (a) Mg is oxidized and Mg is the reducing agent; Ni is reduced and NiCl2 is the oxidizing agent
(b) P is oxidized and PCl3 is the reducing agent; Cl is reduced and Cl2 is the oxidizing agent
(c) Zn is oxidized and Zn is the reducing agent; H is reduced and H2SO4 is the oxidizing agent
(d) Cu is oxidized and Cu is the reducing agent; N is reduced and HNO3 is the oxidizing agent - (a) 2 Al(s) + 3 F2(g) → 2 AlF3(s)
(b) 2 Al(s) + 3 CuBr2(aq) → 2 AlBr3(aq) + 3 Cu(s)
(c) P4(s) + 5 O2 (g) → 2 P2O5(s) - (a) 2 K(s) + 2 H2O(ℓ) ⟶ 2 KOH(aq) + H2(g); K is the reducing agent, H2O is the oxidizing agent
(b) Ba(s) + 2 HBr(aq) ⟶ BaBr2(aq) + H2(g); Ba is the reducing agent, HBr is the oxidizing agent
(c) Sn(s) + 2 I2(s) ⟶ SnI4(s); Sn is the reducing agent, I2 is the oxidizing agent - False
- Only reaction (b) will occur.
- D
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

