M12Q0: Drawing Conventions
Learning Objectives
- Use and understand different representations of molecules: ball and stick model, space-filling model, molecular formula, Lewis structure, structural formula, condensed structural formula, dash-wedge formula, and line formula.
| Lewis Structures | Dash-Wedge Structures | Bond Line Structures | Condensed Formula |
In organic molecules, nearly all bonds involve the sharing of valence shell electrons between atoms in covalent bonds. In this section, we will explore the different conventions used to represent molecules and their valence shell electrons and chemical bonds.
Two organic molecules – butane (C4H10) and butanoic acid (C4H8O2) – will be used throughout this section to demonstrate some of the more prominent drawing conventions.
Lewis Structures
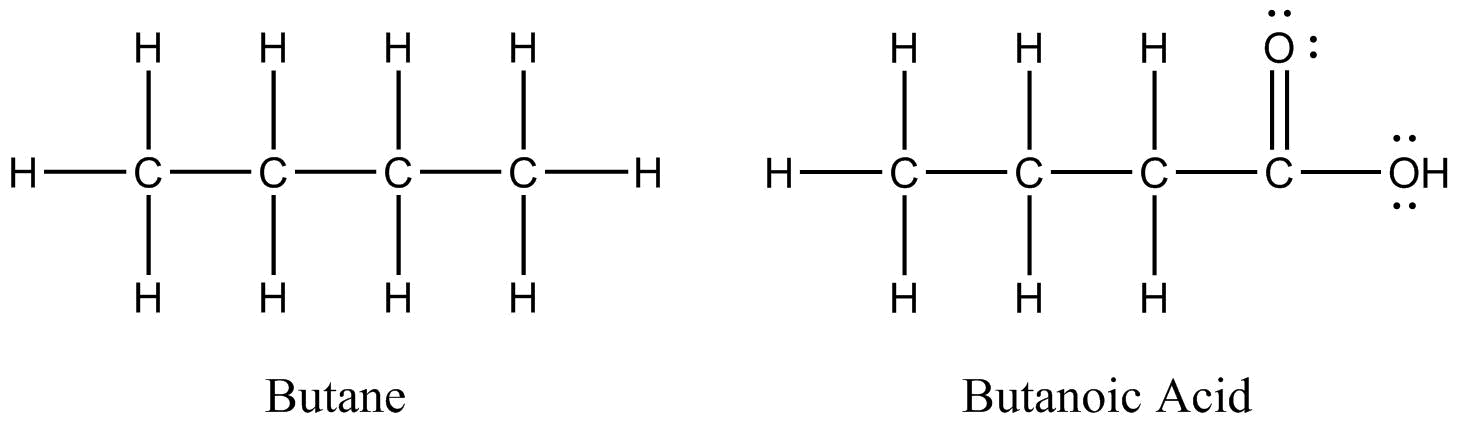
Lewis structures show all atoms, bonds, and non-bonding electron pairs.
Creating Lewis Structures:
- Determine the total number of valence (outer shell) electrons. For cations, subtract one electron for each positive charge. For anions, add one electron for each negative charge.
- Draw a skeleton structure of the molecule or ion, either arranging the atoms around a single central atom or in a series of connected central atoms. Connect remaining atoms to the central atom(s) with single bonds.
- Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen), completing an octet around each atom.
- If necessary, place all remaining electrons on the central atom(s).
- If central atoms lack a full octet, rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets.
Lewis Structure Tips and Cautions:
- Atoms that form the most bonds will usually be central atoms.
- Hydrogen is NEVER a central atom. Hydrogen will only form ONE bond. Hydrogen is NEVER a “bridge”, between two atoms.
- Carbon, nitrogen, and oxygen will never have more than a total of eight valence electrons, bonding and non-bonding. Do not form molecules where carbon has five bonds.
- In organic molecules, atoms bond with some predictability: carbon will usually form four total bonds, nitrogen will usually form three bonds, and oxygen will usually form two bonds. The predicted numbers of bonds may be all single bonds or some combination of single, double and triple bonds. For example:
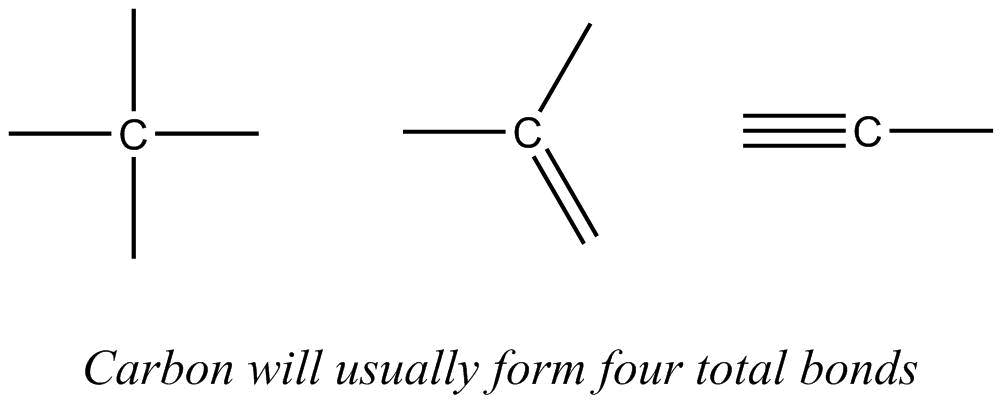
Lewis structures can be very useful. By showing all electron domains (bonding or non-bonding), they allow for easy determination of electron and molecular geometry. It is relatively simple to tell if the ‘octet rule’ is adhered to and to calculate formal charges. However, there are also some drawbacks as they quickly become complicated and unwieldy as molecules become larger. They are also not 3-dimensional representations.
Dash-Wedge Structures
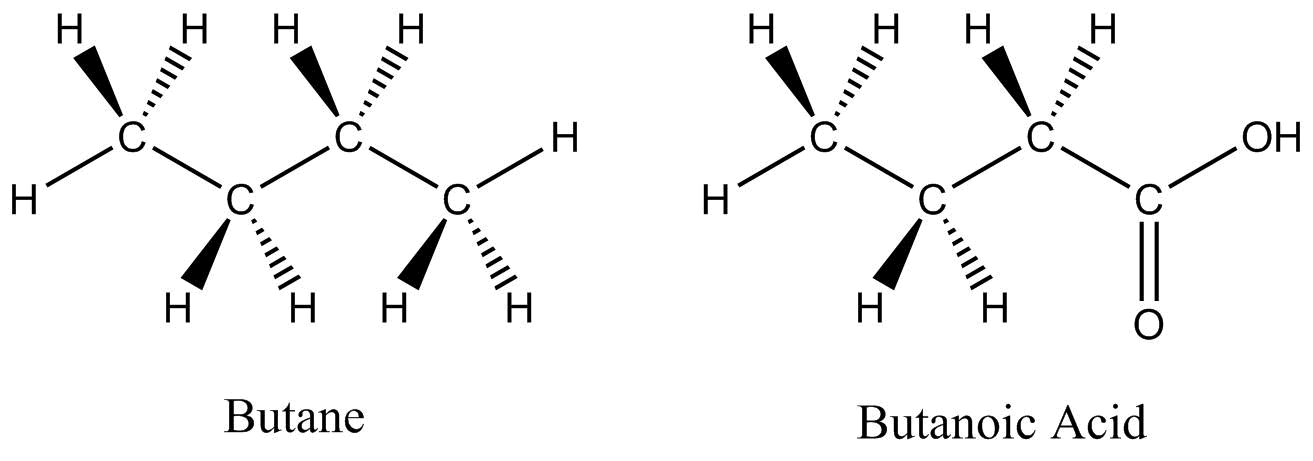
Dash-wedge structures are three-dimensional representations of molecules.
Creating Dash-Wedge Structures:
- It may be helpful to sketch a Lewis structure before drawing a dash-wedge structure in order to establish atom connectivity, bonding, and predicted bond angles.
- In dash-wedge structures, solid lines are used to show the backbone of the chemical structure; bonds and atoms connected by solid lines are in the same plane. For the backbone choose the longest chain of connected atoms.
- Wedges are used to represent bonds and atoms coming forward from the backbone plane. Dashes are used to represent bonds and atoms going backward from the backbone plane.
- In dash-wedge structures, all atoms and bonds are shown. Non-bonding electron pairs are generally not shown, except when useful for understanding a molecules properties and behavior.
Dash-Wedge Tips and Cautions:
- If you have access to a molecular modeling kit, it is very helpful to build a few molecules and sketch them to become comfortable with this representation.
- These representations are meant to replicate predicted bond angles.
- There are many ways to draw these, but remember, the representations are a communication tool, so strive for consistency and uniformity when drawing.
The main benefit of using dash-wedge structures is that they more accurately show three-dimensional structure; however, they can become unmanageable as molecules become larger and too time-consuming to draw.
Bond Line Structures
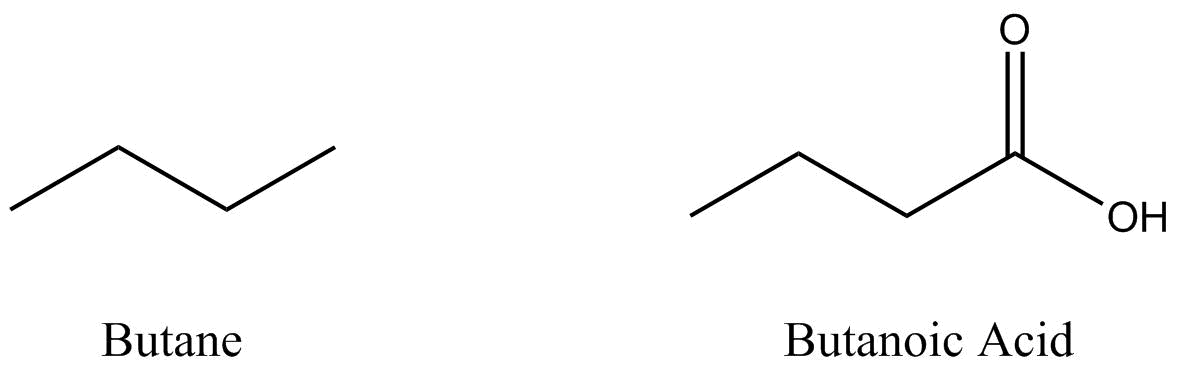
Bond line structures are simplified representations of molecules.
Creating Bond Line Structures:
- Single lines are used to show single covalent bonds (with two parallel lines for a double bond and three parallel lines for a triple bond) as in previous representations.
- Carbon atoms and hydrogen atoms bonded to carbon are NOT shown.
- Non-carbon atoms and hydrogen atoms attached to non-carbon atoms are shown.
- Hydrogen atoms that are a defined part of a functional group (such as in aldehydes) are typically shown.
- Where no atom is shown, vertices and ends of lines represent carbon atoms. Carbon atoms with incomplete octets in the structure are assumed to be completed with bonds to hydrogen atoms.
- Non-bonding electron pairs are generally not shown, except when useful for understanding a molecule’s properties and behavior.
Bond Line Structure Tips and Cautions:
- Make sure you correctly count your carbons in the chain. The end points and each intersection are a single carbon.
Line structures are easier to draw than the previously discussed structures, especially for complicated molecules; however, they do not accurately represent three-dimensional structure and can be difficult to interpret at first glance.
Example 1
Drawing Bond Line Structures
Draw the bond line structures for these two molecules:
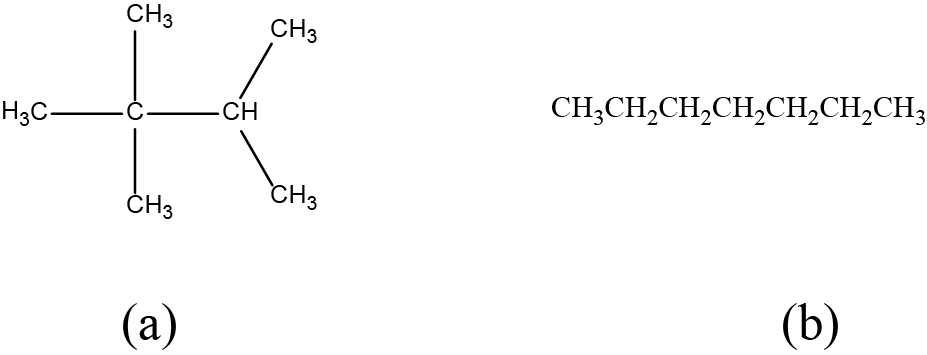
Solution
Each carbon atom is converted into the end of a line or the place where lines intersect. All hydrogen atoms attached to the carbon atoms are left out of the structure (although we still need to recognize they are there):
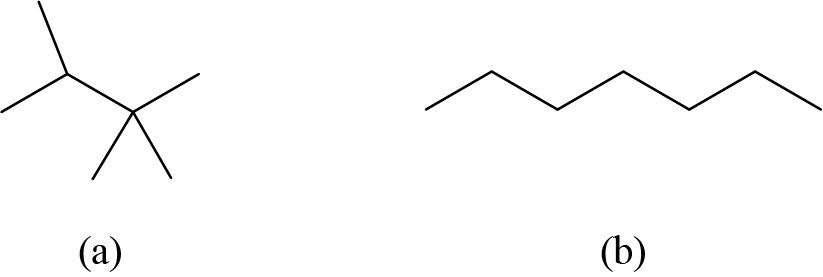
Check Your Learning
Draw the bond line structures for these two molecules:
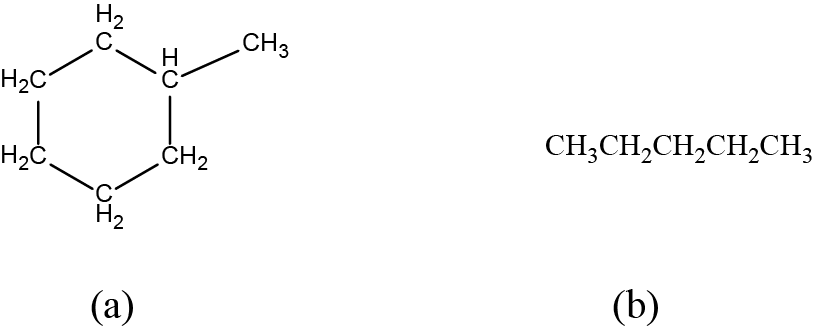
Answer:
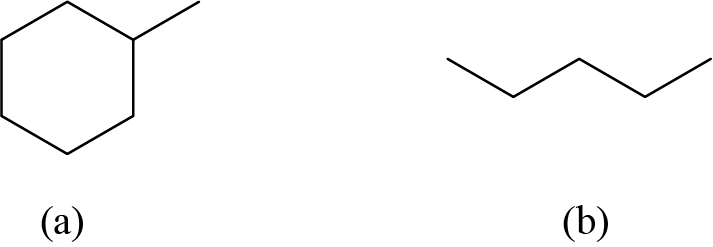
Example 2
Interpreting Bond Line Structures
Identify the chemical formula of the molecule represented here:
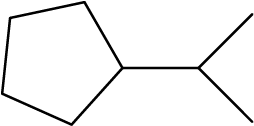
Solution
There are eight places where lines intersect or end, meaning that there are eight carbon atoms in the molecule. Since we know that carbon atoms tend to make four bonds, each carbon atom will have the number of hydrogen atoms that are required for four bonds.
Location of the hydrogen atoms:
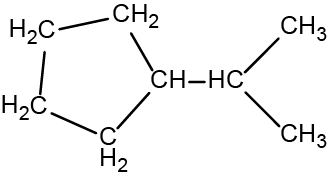
This compound contains 16 hydrogen atoms for a molecular formula of C8H16.
Check Your Learning
Identify the chemical formula of the molecule represented here:

Answer:
C9H20
Condensed Formulas

Condensed formulas show all atoms but leave out most or all bonds. Parentheses are used to show groups of atoms (branches) bonded to the nearest non-hydrogen atom to the left.
Writing Condensed Formulas:
- Determine the longest chain of carbons.
- Beginning with the first carbon, write C and then each atom or group of atoms attached to that carbon, except for the next carbon in the chain.
- Groups of atoms should be enclosed in parenthesis. Repeat this with each carbon in the longest chain.
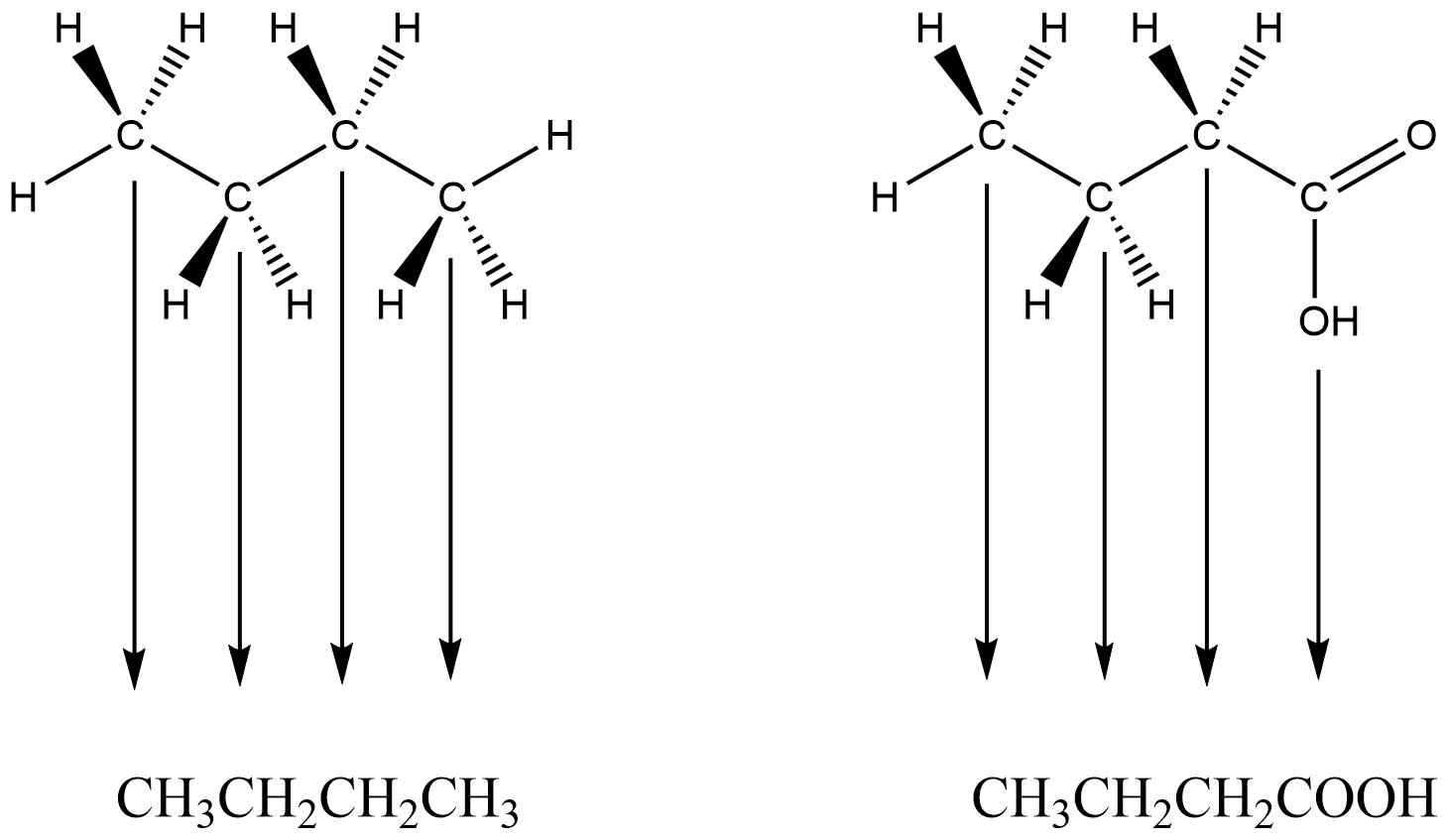
Condensed Formula Tips and Cautions:
- Condensed formulas can be found written from the left end of the longest carbon chain to the right end or visa versa, so there may be multiple ways to write a condensed formula for a given molecule.
- Multiple bonds are not shown but assumed when there are fewer than four bonds shown to a carbon.
- Functional groups may make condensed structures difficult to interpret.
- There are times when a structural component may be added to a condensed formula to aid interpretation; for example, a “=” may be placed between carbons joined by a double bond.
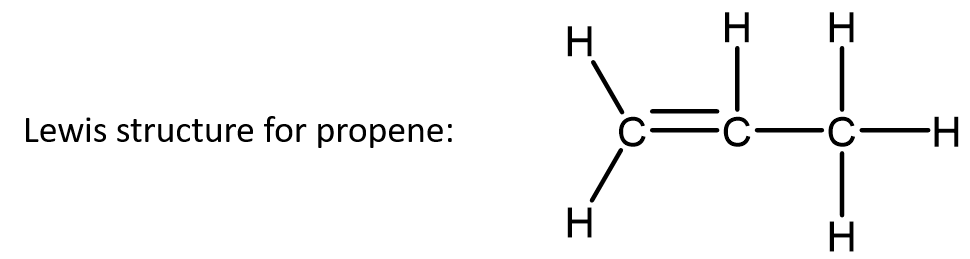 The condensed formula for propene (C3H6) may be written as CH2CHCH3 or CH2=CHCH3. The double bond is clear in the Lewis structure shown. The double bond’s presence is inferred in the first condensed formula because the first and second carbons only have three atoms attached, and if these were all attached with single bonds, then these carbons would have incomplete octets. The second condensed formula adds a structural component to clearly indicate a double bond is present.
The condensed formula for propene (C3H6) may be written as CH2CHCH3 or CH2=CHCH3. The double bond is clear in the Lewis structure shown. The double bond’s presence is inferred in the first condensed formula because the first and second carbons only have three atoms attached, and if these were all attached with single bonds, then these carbons would have incomplete octets. The second condensed formula adds a structural component to clearly indicate a double bond is present.
Condensed formulas allow bonding and atomic arrangements to be inferred from how the formula is written from left to right. They do not require special graphics programs to create them, but it is possible to write multiple condensed formulas for the same molecule. They can also be complicated and do not depict three-dimensional structure.
Key Concepts and Summary
Uniform representations of molecules is extremely important when using chemistry across different sciences and languages. The process for naming and drawing molecules is quite straightforward, although it requires a basic understanding of organic chemistry. In this section, we looked at the four primary ways of drawing a molecule, with the most common way being the bond-line structure. Feeling comfortable with all four methods, but especially bond-line structures, is of fundamental importance to understanding organic chemistry.
Glossary
- bond-line structure
- simplified representations of molecules showing all non-C,H atoms and non C-H bonds
- condensed formula
- show all atoms but leave out most (or all) bonds. Parantheses are used to show groups of atoms (branches) bonded to the nearest non-hydrogen atom to the left
- dash-wedge structure
- three-dimensional represenations of molecules showing all atoms and bonds
- Lewis structure
- shows all atoms, bonds, and non-bonding electron pairs
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

