M12Q6: Condensation and Hydrolysis Reactions
Learning Objectives
- Identify condensation reactions, and write balanced chemical reactions for the formation of esters and carboxamides.
| Condensation of Alcohols | Condensation of Amines | - Identify and write balanced chemical reactions for the acid-catalyzed hydrolysis of esters and amides.
| Hydrolysis of Esters | Hydrolysis of Amides |
Condensation reactions are reactions between two molecules that create one large and one small (usually H2O) molecule. For this module, we will be learning about two condensation reactions: one which creates esters and one which creates amides. Hydrolysis reactions are just the opposite of condensation reactions. In a hydrolysis reaction, we take one large and one small molecule to create two new molecules.
Condensation to Create Esters
Esters are produced by the reaction of carboxylic acids with alcohols with an acid catalyst (usually H2SO4). In these reactions, the -OH group from the carboxylic acid combines with the -H from the alcohol to produce water. The resulting -OR group from the alcohol combines with the carboxylic acid remnant to create an ester group, as can be seen in Figure 1.
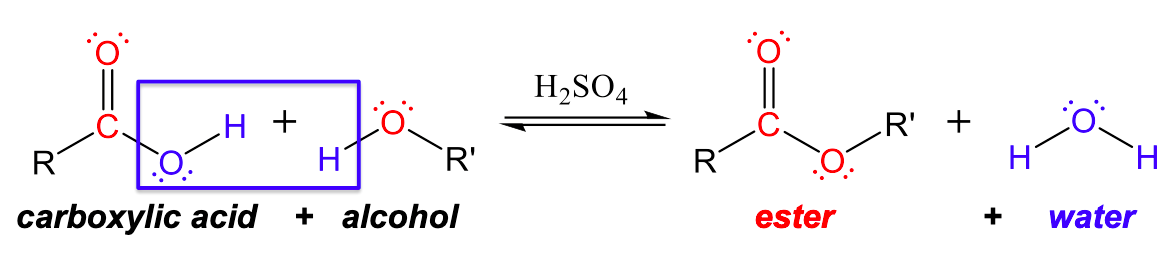
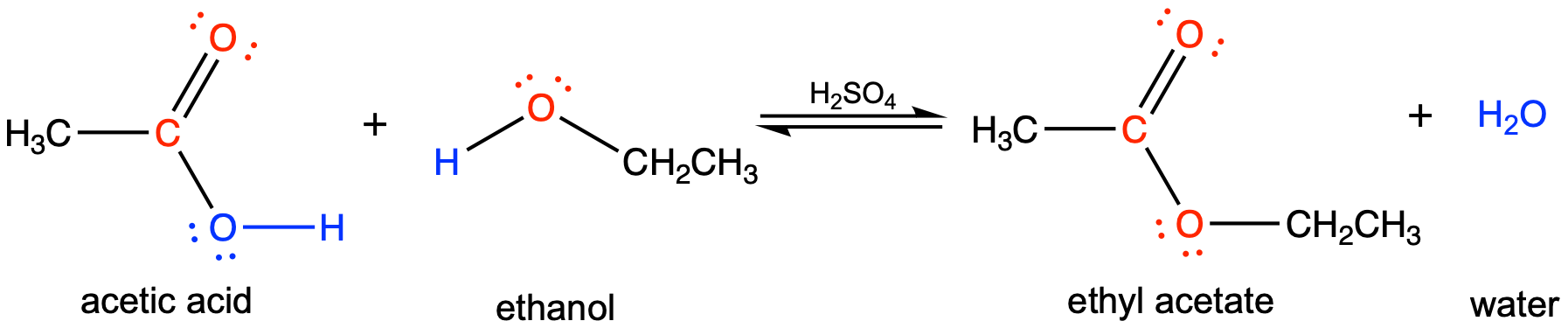
For example, the ester ethyl acetate, CH3CO2CH2CH3, is formed when acetic acid reacts with ethanol:
Hydrolysis of Esters
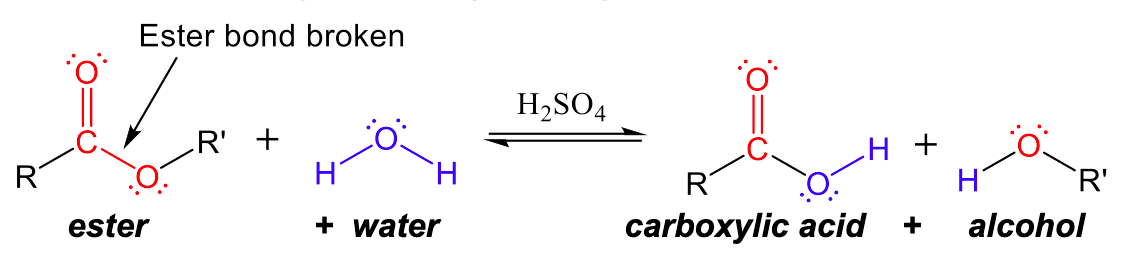
Ester functional groups can also be broken back into carboxylic acids and alcohols with the opposite, hydrolysis reaction. In Figure 2, we can see that the ester bond is broken between the C-O bond. The carbonyl (C=O) carbon forms a covalent bond with -OH from water to create a carboxylic acid group, and the -OR group that broke off of the ester combines with the -H of water to create an alcohol.
Condensation to Create Amides
Amides can be produced when carboxylic acids react with amines or ammonia through a condensation reaction (Figure 3). A water molecule is eliminated from the reaction, and the amide is formed from the remaining pieces of the carboxylic acid and the amine (note the similarity to formation of an ester from a carboxylic acid and an alcohol discussed previously):
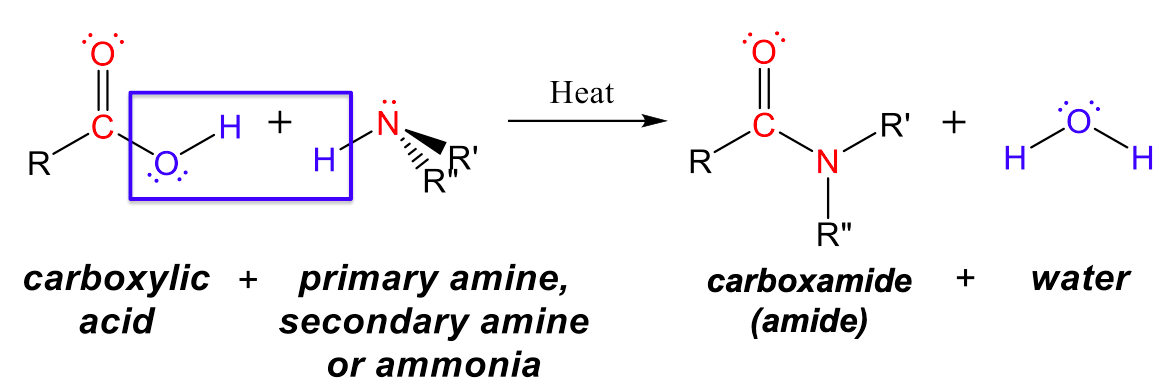
The reaction between amines and carboxylic acids to form amides is biologically important. It is through this reaction that amino acids (molecules containing both amine and carboxylic acid substituents) link together in a polymer to form proteins.
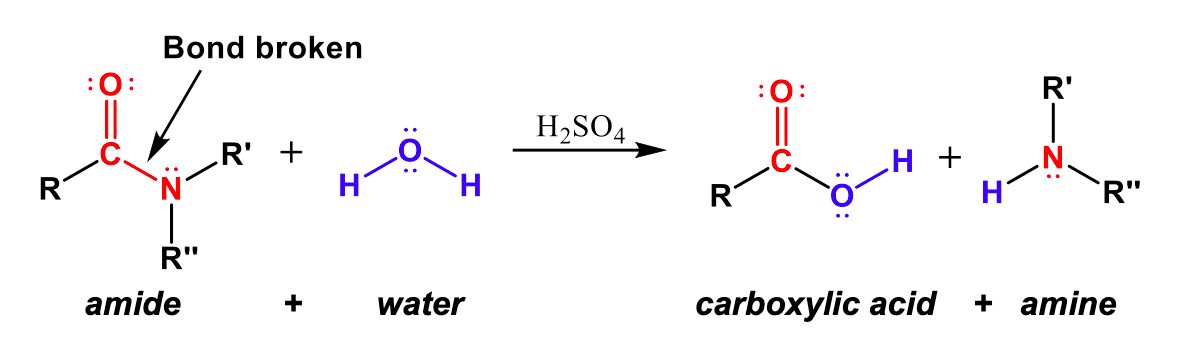
Hydrolysis of Amides
Amide functional groups can also be broken back into carboxylic acids and amines with the opposite, hydrolysis reaction. In Figure 4, we can see that the amide bond is broken between the C-N bond. The carbonyl (C=O) carbon forms a covalent bond with -OH from water to create a carboxylic acid group, and the -NRR” group that broke off of the amide combines with the -H of water to create an amine.
Chemistry in Real Life: Proteins and Enzymes
Proteins are large biological molecules made up of long chains of smaller molecules called amino acids. Organisms rely on proteins for a variety of functions—proteins transport molecules across cell membranes, replicate DNA, and catalyze metabolic reactions, to name only a few of their functions. The properties of proteins are functions of the combination of amino acids that compose them and can vary greatly. Interactions between amino acid sequences in the chains of proteins result in the folding of the chain into specific, three-dimensional structures that determine the protein’s activity.
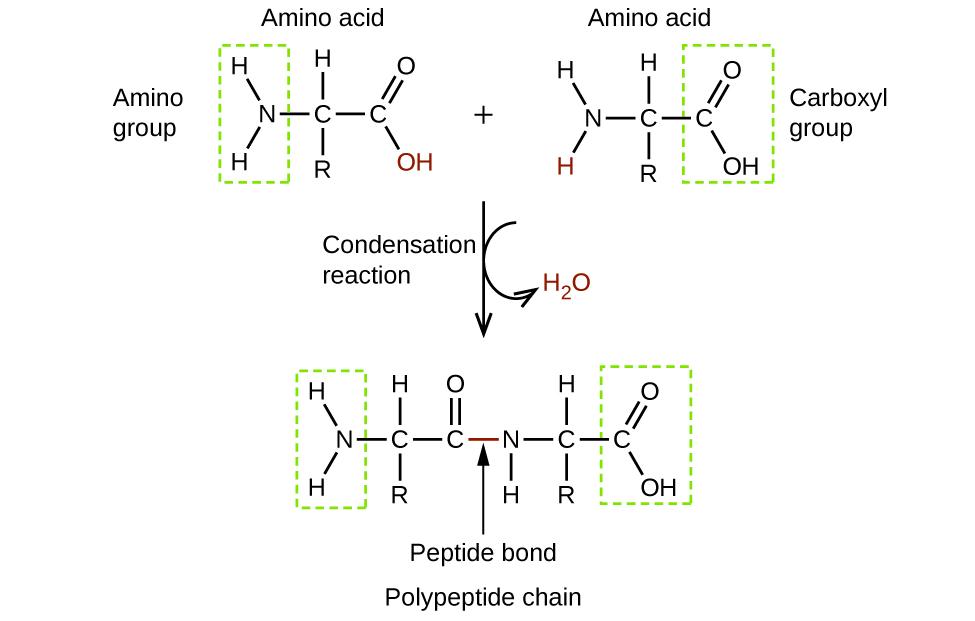
Amino acids are organic molecules that contain an amine functional group (–NH2), a carboxylic acid functional group (–COOH), and a side chain (that is specific to each individual amino acid). Most living things build proteins from the same 20 different amino acids. Amino acids connect by the formation of a peptide bond, which is a covalent bond formed between two amino acids when the carboxylic acid group of one amino acid reacts with the amine group of the other amino acid. The formation of the bond results in the production of a molecule of water (in general, reactions that result in the production of water when two other molecules combine are referred to as condensation reactions). The resulting bond—between the carbonyl group carbon atom and the amine nitrogen atom–is called a peptide link or peptide bond. Since each of the original amino acids has an unreacted group (one has an unreacted amine and the other an unreacted carboxylic acid), more peptide bonds can form to other amino acids, extending the structure. (Figure 5) A chain of connected amino acids is called a polypeptide. Proteins contain at least one long polypeptide chain.
Enzymes are large biological molecules, mostly composed of proteins, which are responsible for the thousands of metabolic processes that occur in living organisms. Enzymes are highly specific catalysts; they speed up the rates of certain reactions. Enzymes function by lowering the activation energy of the reaction they are catalyzing, which can dramatically increase the rate of the reaction. Most reactions catalyzed by enzymes have rates that are millions of times faster than the noncatalyzed version. Like all catalysts, enzymes are not consumed during the reactions that they catalyze. Enzymes do differ from other catalysts in how specific they are for their substrates (the molecules that an enzyme will convert into a different product). Each enzyme is only capable of speeding up one or a few very specific reactions or types of reactions. Since the function of enzymes is so specific, the lack or malfunctioning of an enzyme can lead to serious health consequences. One disease that is the result of an enzyme malfunction is phenylketonuria. In this disease, the enzyme that catalyzes the first step in the degradation of the amino acid phenylalanine is not functional (Figure 6). Untreated, this can lead to an accumulation of phenylalanine, which can lead to intellectual disabilities.
Chemistry in Real Life: Kevlar
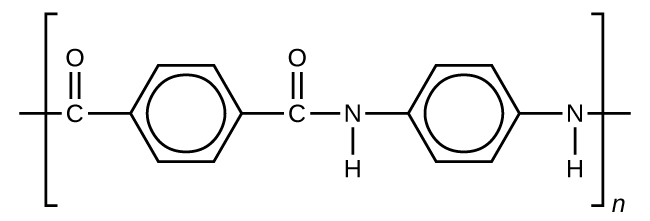
Kevlar (Figure 7) is a synthetic polymer made from two monomers 1,4-phenylene-diamine and terephthaloyl chloride (Kevlar is a registered trademark of DuPont). Kevlar’s first commercial use was as a replacement for steel in racing tires. Kevlar is typically spun into ropes or fibers. The material has a high tensile strength-to-weight ratio (it is about 5 times stronger than an equal weight of steel), making it useful for many applications from bicycle tires to sails to body armor.
The material owes much of its strength to hydrogen bonds between polymer chains (refer back to the chapter on intermolecular interactions). These bonds form between the carbonyl group oxygen atom (which has a partial negative charge due to oxygen’s electronegativity) on one monomer and the partially positively charged hydrogen atom in the N–H bond of an adjacent monomer in the polymer structure (see dashed line in Figure 8). There is additional strength derived from the interaction between the unhybridized p orbitals in the six-membered rings, called aromatic stacking.
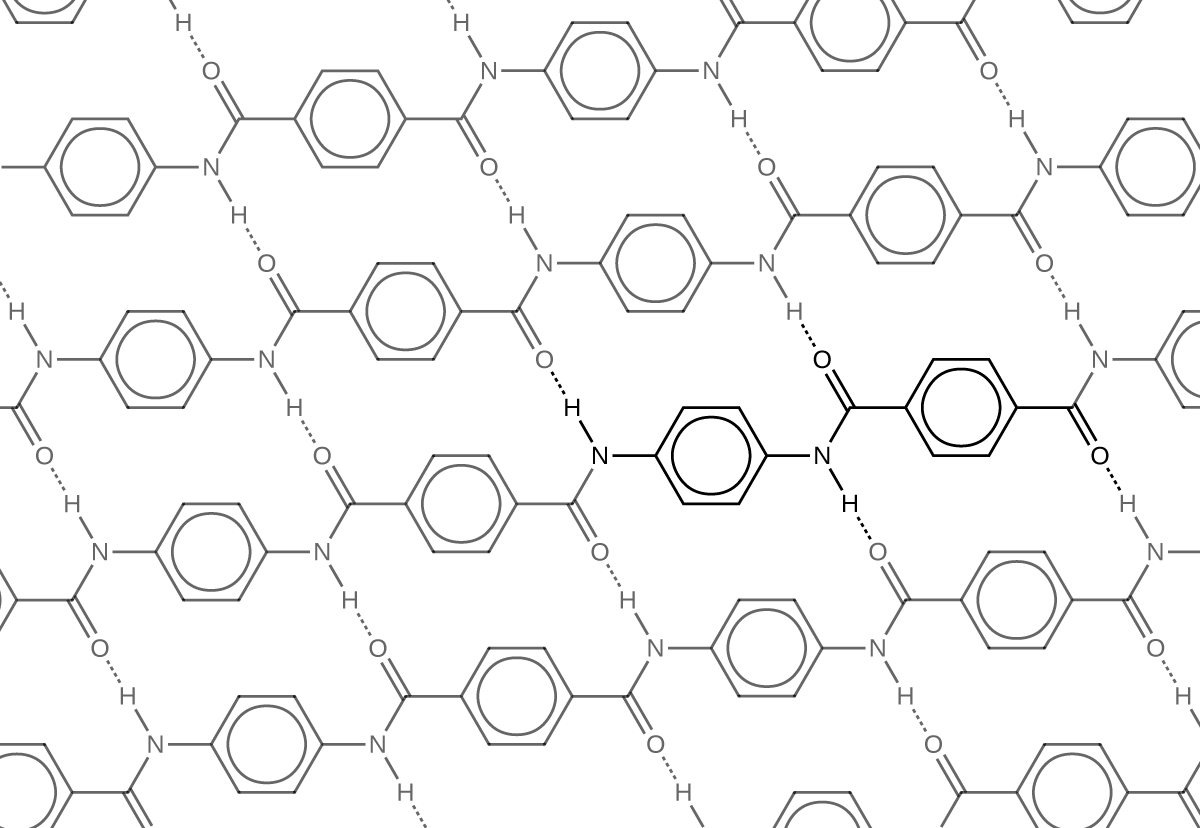
Kevlar may be best known as a component of body armor, combat helmets, and face masks. Since the 1980s, the US military has used Kevlar as a component of the PASGT (personal armor system for ground troops) helmet and vest. Kevlar is also used to protect armored fighting vehicles and aircraft carriers. Civilian applications include protective gear for emergency service personnel such as body armor for police officers and heat-resistant clothing for fire fighters. Kevlar based clothing is considerably lighter and thinner than equivalent gear made from other materials (Figure 9).

In addition to its better-known uses, Kevlar is also often used in cryogenics for its very low thermal conductivity (along with its high strength). Kevlar maintains its high strength when cooled to the temperature of liquid nitrogen (–196 °C).
Key Concepts and Summary
Each functional group is used to create other functional groups and created by a variety of reactions. Two types of reaction, condensation and hydrolysis, are introduced in this section. For simplicity, condensation reactions come in two types:
carboxylic acid + alcohol ⇌ ester + H2O (catalyst: H2SO4)
carboxylic acid + amine ⇌ amide + H2O (catalyst: heat)
Hydrolysis reactions are the opposite of condensation reactions:
ester + H2O ⇌ carboxylic acid + alcohol (catalyst: H2SO4)
amide + H2O ⇌ carboxylic acid + amine (catalyst: H2SO4)
Glossary
- condensation reaction
- a reaction between two molecules that create one large and one small (usually H2O) molecule
- hydrolysis reaction
- a reaction between two molecules (one usually H2O) to create two different molecules; the opposite of a condensation reaction
Chemistry End of Section Exercises
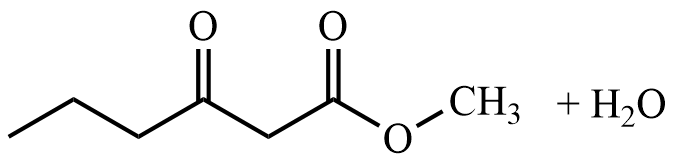
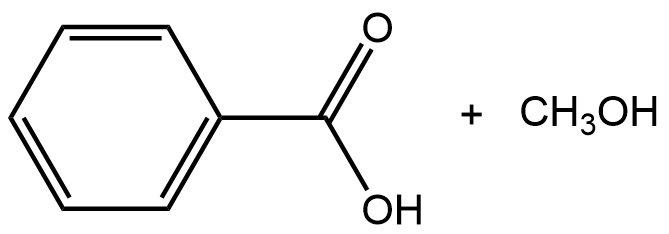
- What are the reactant(s) that would form the following products? Draw the reactant(s) of the reaction using any correct structural formula(s).
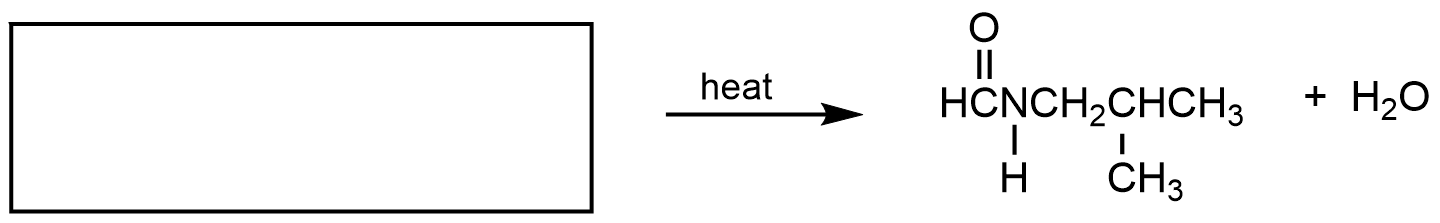
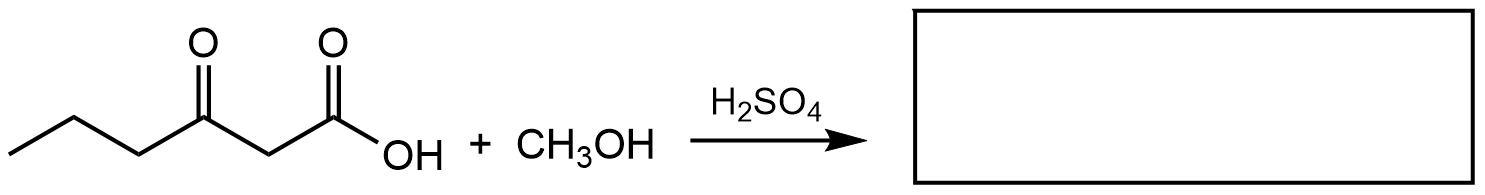
- What are the product(s) of the following reaction? Draw the product(s) of the reaction using any correct structural formula(s). If no reaction occurs, write “no reaction”.
- Draw any correct structural formula for the product(s). If no reaction occurs, write “no reaction”.
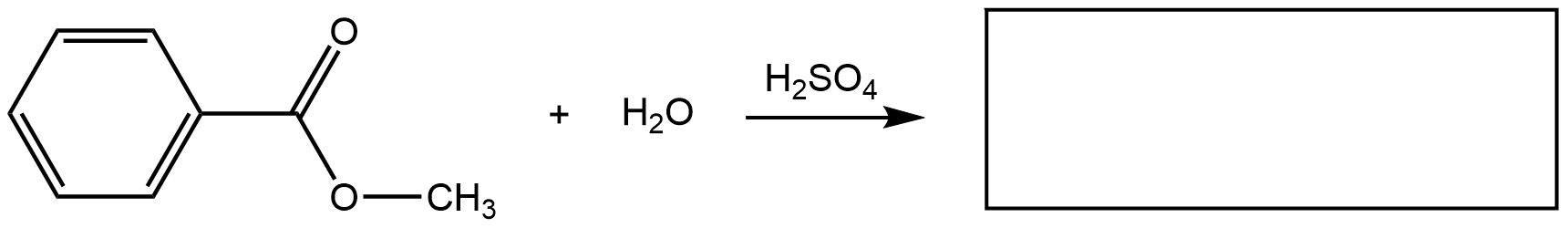
Answers to Chemistry End of Section Exercises
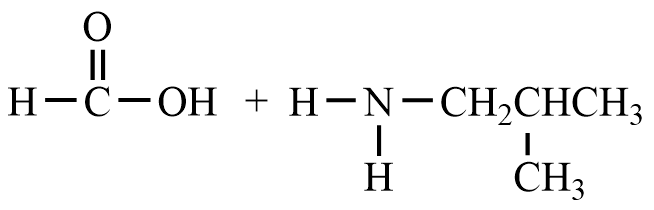
Left-click here to watch Exercise 1 problem solving video.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

