M15Q6: Polyprotic Acids
Learning Objectives
- Determine the pH of an aqueous solution containing a polyprotic acid or base.
- Write the chemical reaction corresponding to a given equilibrium constant for polyprotic acids and their conjugate bases (Ka, Kb, Kw, Ka1, Ka2, Ka3, Kb1, Kb2, Kb3).
| Key Concepts and Summary | Glossary | End of Section Exercises |
We can classify acids by the number of protons per molecule that they can give up in a reaction. Acids such as HCl, HNO3, and HCN that contain one acidic hydrogen atom in each molecule are called monoprotic acids. Their reactions with water are:
HCl(aq) + H2O(ℓ) → H3O+(aq) + Cl–(aq)
HNO3(aq) + H2O(ℓ) → H3O+(aq) + NO3–(aq)
HCN(aq) + H2O(ℓ) ⇌ H3O+(aq) + CN–(aq)
Even though it contains four hydrogen atoms, acetic acid, CH3COOH, is also monoprotic because only the hydrogen atom from the carboxyl group (-COOH) reacts with bases:
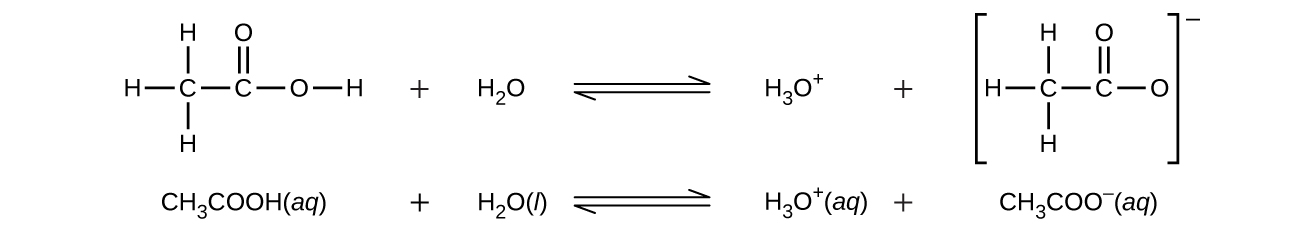
Similarly, monoprotic bases are bases that will accept a single proton.
Diprotic acids contain two acidic hydrogen atoms per molecule; ionization of such acids occurs in two steps. The first ionization always takes place to a greater extent than the second ionization. For example, sulfuric acid, a strong acid, ionizes as follows:
| First ionization: | H2SO4(aq) + H2O(ℓ) ⇌ H3O+(aq) + HSO4–(aq) | Ka1 = > 102 |
| Second ionization: | HSO4–(aq) + H2O(ℓ) ⇌ H3O+(aq) + SO42-(aq) | Ka2 = 1.1 × 10-2 |
This stepwise ionization process occurs for all polyprotic acids, or acids that contain more than one acidic hydrogen. When we make a solution of a weak diprotic acid, we get a solution that contains a mixture of acids. Carbonic acid, H2CO3, is an example of a weak diprotic acid. The first ionization of carbonic acid yields hydronium ions and bicarbonate ions in small amounts, resulting in:
KH2CO3 = Ka1
First ionization:
H2CO3(aq) + H2O(ℓ) ⇌ H3O+(aq) + HCO3–(aq) KH2CO3 = ![]() = 4.3 × 10-7
= 4.3 × 10-7
The bicarbonate ion can also act as an acid. It ionizes and forms hydronium ions and carbonate ions in even smaller quantities, resulting in:
KHCO3– = Ka2
Second ionization:
HCO3–(aq) + H2O(ℓ) ⇌ H3O+(aq) + CO32-(aq) KHCO3– = ![]() = 4.7 × 10-11
= 4.7 × 10-11
KH2CO3 is larger than KHCO3– by a factor of 104, so H2CO3 is the dominant producer of hydronium ion in the solution. This means that little of the HCO3– formed by the ionization of H2CO3 ionizes to give hydronium ions (and carbonate ions), and the concentrations of H3O+ and HCO3– are practically equal in a pure aqueous solution of H2CO3.
If the first ionization constant of a weak diprotic acid is larger than the second by a factor of at least 20, it is appropriate to treat the first ionization separately and calculate concentrations resulting from it before calculating concentrations of species resulting from subsequent ionization. This can simplify our work considerably because we can determine the concentration of H3O+ and the conjugate base from the first ionization, then determine the concentration of the conjugate base of the second ionization in a solution with concentrations determined by the first ionization.
Example 1
Ionization of a Diprotic Acid
When we buy soda water (carbonated water), we are buying a solution of carbon dioxide in water. The solution is acidic because CO2 reacts with water to form carbonic acid, H2CO3. What are [H3O+], [HCO3–], and [CO32-] in a saturated solution of CO2 with an initial [H2CO3] = 0.033 M?
H2CO3(aq) + H2O(ℓ) ⇌ H3O+(aq) + HCO3–(aq) Ka1 = 4.3 × 10-7
HCO3–(aq) + H2O(ℓ) ⇌ H3O+(aq) + CO32–(aq) Ka2 = 4.7 × 10-11
Solution
As indicated by the ionization constants, H2CO3 is a much stronger acid than HCO3–, so H2CO3 is the dominant producer of hydronium ion in solution. Thus there are two parts in the solution of this problem: (1) Using the customary four steps, we determine the concentration of H3O+ and HCO3– produced by ionization of H2CO3. (2) Then we determine the concentration of CO32- in a solution with the concentration of H3O+ and HCO3– determined in (1). To summarize:
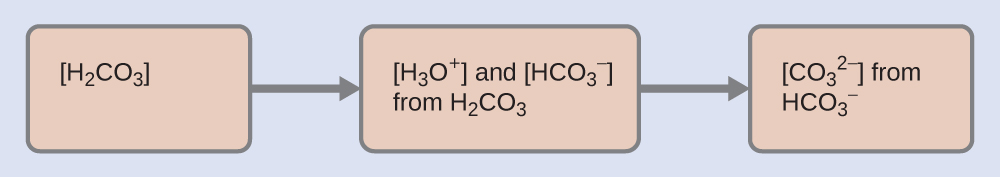
- Determine the concentrations of H3O+ and HCO3–.
H2CO3(aq) + H2O(ℓ) ⇌ H3O+(aq) + HCO3–(aq) Ka1 = 4.3 × 10-7
As for the ionization of any other weak acid:
An abbreviated table of changes and concentrations shows:
H2CO3(aq) + H2O(ℓ) ⇌ H3O+(aq) + HCO3–(aq) I 0.033 – 0 0 C –x – +x +x E 0.033 – x – x x Substituting the equilibrium concentrations into the equilibrium gives us:
KH2CO3 =
![Rendered by QuickLaTeX.com \dfrac{[\text{H}_{3}\text{O}^{+}][\text{HCO}_{3}^{-}]}{[\text{H}_{2}\text{CO}_{3}]} = \dfrac{(x)(x)}{0.033 - x}](https://wisc.pb.unizin.org/app/uploads/quicklatex/quicklatex.com-f986fec95792ad350401bb02707186dd_l3.png) = 4.3 × 10-7
= 4.3 × 10-7Solving the preceding equation making the assumption (0.033 – x ≈ 0.033) gives:
x = 1.2 × 10-4 M
Thus:
[H2CO3] = 0.033 – x = 0.033 M
[H3O+] = [HCO3–] = 1.2 × 10-4 M - Determine the concentration of CO32- in a solution at equilibrium with [H3O+] and [HCO3–] both equal to 1.2 ×10−4 M.
HCO3–(aq) + H2O(ℓ) ⇌ H3O+(aq) + CO32–(aq)
KHCO3– =
![Rendered by QuickLaTeX.com \dfrac{[\text{H}_{3}\text{O}^{+}][\text{CO}_{3}^{2-}]}{[\text{HCO}_{3}^{-}]} = \dfrac{(1.2 \times 10^{-4})[\text{CO}_{3}^{2-}]}{1.2 \times 10^{-4}}](https://wisc.pb.unizin.org/app/uploads/quicklatex/quicklatex.com-3c78006798192e09fb224b3fdda1c9c1_l3.png) = 4.7 × 10-11
= 4.7 × 10-11[CO32-] =
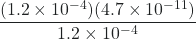 = 4.7 × 10-11 M
= 4.7 × 10-11 M
To summarize: In part 1 of this example, we found that the H2CO3 in a 0.033-M solution ionizes slightly and at equilibrium [H2CO3] = 0.033 M; [H3O+] = 1.2 × 10-4 M; and [HCO3–] = 1.2 × 10-4 M. In part 2, we determined that [CO32-] = 4.7 × 10-11 M.
Check Your Learning
The concentration of H2S in a saturated aqueous solution at room temperature is approximately 0.1 M. Calculate [H3O+], [HS−], and [S2−] in the solution:
H2S(aq) + H2O(ℓ) ⇌ H3O+(aq) + HS–(aq) Ka1 = 1.0 × 10-7
HS–(aq) + H2O(ℓ) ⇌ H3O+(aq) + S2-(aq) Ka2 = 1.0 × 10-19
Answer:
[H2S] = 0.1 M; [H3O+] = [HS−] = 1.0 × 10-4 M; [S2−] = 1 × 10−19 M
We note that the concentration of the sulfide ion is the same as Ka2. This is due to the fact that each subsequent dissociation occurs to a lesser degree (as acid gets weaker).
A triprotic acid is an acid that has three dissociable protons that undergo stepwise ionization: Phosphoric acid is a typical example:
| First ionization: | H3PO4(aq) + H2O(ℓ) ⇌ H3O+(aq) + H2PO4–(aq) | Ka1 = 7.2 × 10-3 |
| Second ionization: | H2PO4–(aq) + H2O(ℓ) ⇌ H3O+(aq) + HPO42-(aq) | Ka2 = 6.3 × 10-8 |
| Third ionization: | HPO42-(aq) + H2O(ℓ) ⇌ H3O+(aq) + PO43-(aq) | Ka3 = 4.6 × 10-13 |
Polyprotic bases can accept more than one hydrogen ion in solution. The carbonate ion is an example of a diprotic base, since it can accept up to two protons. Solutions of alkali metal carbonates are quite alkaline, due to the reactions:
CO32-(aq) + H2O(ℓ) ⇌ HCO3–(aq) + OH–(aq)
HCO3–(aq) + H2O(ℓ) ⇌ H2CO3(aq) + OH–(aq)
Key Concepts and Summary
In this section, we look at acids and bases that can donate or accept multiple protons. Ionizations are stepwise and we write equilibrium reactions and expressions for each proton being donated or accepted, one at a time. You might notice that Ka1 > Ka2 > Ka3 >… since each successive proton is harder to remove. The same is true for Kb values, where Kb1 > Kb2 > Kb3. In this course, we approximate the number of hydronium or hydroxide ions in the solution from being only from the first Ka or Kb equilibrium reaction.
Glossary
- monoprotic acid
- a molecule that contains only one acidic hydrogen atom
- polyprotic acid
- a molecule that contains more than one acidic hydrogen
- polyprotic base
- a molecule that can accept more than one hydrogen in solution
- stepwise ionization
- the concept that in acid/base reactions, protons are donated or accepted one at a time
Chemistry End of Section Exercises
- Which of the following concentrations would be practically equal in a calculation of the equilibrium concentrations in a 0.134 M solution of H2CO3, a diprotic acid: [H3O+], [OH–], [H2CO3], [HCO3–], [CO32-]? No calculations are needed to answer this question.
- Citric acid, C3H5(COOH)3, is a polyprotic acid, with Ka1 = 1.4 × 10-3, Ka2 = 4.5 × 10-5, and Ka3 = 1.5 × 10-6.
- What species are created with each removal of an acidic hydrogen? (Look at the way the condensed formula is written for a clue.)
- What are the Ka and Kb of the species present after the second H+ is removed?
- Will this species be acidic, neutral or basic when dissolved in water?
- What are the Ka and Kb of HTeO3–? (H2TeO3 is a polyprotic acid and has Ka1 = 7.1 × 10-7, Ka2 = 4.0 × 10-9)
- Will the following species dissolved in water produce an acid, basic, or neutral environment: H2SO3, HSO3–, SO32-? (Ka1 = 1.7 × 10-2, Ka2 = 6.3 × 10-8)
- Will the following species dissolved in water produce an acid, basic, or neutral environment: H2Se, HSe–, Se2-? (Ka1 = 2.0 × 10-4, Ka2 = 1.0 × 10-11)
- From the equilibrium that describes HPO42– dissolving in water and acting as a weak base, identify the two conjugate acid/base pairs.
- HPO42–/PO43– and H3O+/OH–
- HPO42–/HPO43– and H3O+/OH–
- H2PO42–/PO43– and H2O/OH–
- HPO42–/H2PO4– and H2O/OH–
- HPO42–/PO43– and H2O/OH–
- Oxalic acid, H2C2O4, is a diprotic acid with Ka1(H2C2O4) = 5.37 × 10–2 and Ka2(HC2O4–) = 5.42 × 10–8. Which of the following equilibria corresponds to the equilibrium constant Kb2(HC2O4–)?
- H2C2O4 (aq) + OH–(aq) ⇌ HC2O4–(aq) + H2O(ℓ)
- HC2O4–(aq) + H2O(ℓ) ⇌ H2C2O4 (aq) + OH–(aq)
- HC2O4–(aq) + OH–(aq) ⇌ C2O42–(aq) + H2O(ℓ)
- C2O42–(aq) + H2O(ℓ) ⇌ HC2O4–(aq) + OH–(aq)
- 2 HC2O4–(aq) ⇌ H2C2O4 (aq) + C2O42–(aq)
- Arsenic acid, H3AsO4, is a triprotic acid with Ka1 = 2.5 × 10–4, Ka2 = 5.6 × 10–8, and Ka3 = 3 × 10–13.
- Write the chemical reaction for the equilibrium that corresponds to the Ka3 value.
- Write the equilibrium constant expression for Ka3.
Answers to Chemistry End of Section Exercises
- [H3O+] and [HCO3–] are practically equal
- (a) C3H5(COOH)2(COO–); C3H5(COOH)(COO–)2; C3H5(COO–)3
(b) C3H5(COOH)(COO–)2: Ka = 1.5 × 10-6 (which is Ka3); Kb = 2.2 × 10-10 (which is Kw/Ka2)
(c) Ka>Kb so acidic - Ka = 4.0 × 10-9; Kb = 1.4 × 10-8
- H2SO3 = acidic; HSO3– = acidic; SO32- = basic
- H2Se = acidic; HSe– = basic; Se2- = basic
- D
- B
- (a) HAsO42-(aq) + H2O(ℓ) ⇌ AsO43-(aq) + H3O+(aq)
(b)![Rendered by QuickLaTeX.com K_{a3} = \dfrac{[\text{AsO}_4^{3-}][\text{H}_3 \text{O}^+]}{[\text{HAsO}_4^{2-}]}](https://wisc.pb.unizin.org/app/uploads/quicklatex/quicklatex.com-a9bf33d33d724d91255e4401bf62c0b2_l3.png)
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

