M13Q3: Rate Laws and Reaction Order: Determining Rate Laws from Empirical Data; Method of Initial Rates
- Describe why experimentation is required to generate a rate law for a given reaction.
- Evaluate experimental data using the method of initial rates to determine reaction orders, the rate constant, and the rate law for a reaction.
| Key Concepts and Summary | Key Equations | Glossary | End of Section Exercises |
Rate Laws
As described in the previous quanta, the rate of a reaction is affected by the concentrations of reactants. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentration of its reactants.
Consider a general reaction like this:
a A + b B → c C + d D
for which the rate law is:
rate = k[A]m[B]n
in which [A] and [B] represent the molar concentrations of reactants (although it is also possible products and catalysts may be represented in the rate law as well), and k is the rate constant, which is specific for a particular reaction at a particular temperature. The exponents, m and n, are usually positive integers (although it is possible for them to be fractions or negative numbers). These exponents are not necessarily the coefficients from the reaction and should not be assumed to be so. We will cover this more in depth later. The rate constant, k, and the order exponents must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants are changed. The exponents in a rate law describe the effects of the reactant concentrations on the reaction rate and define the reaction order.
If the exponent m is 1, the reaction is first order with respect to A. If m is 2, the reaction is second order with respect to A. If n is 1, the reaction is first order in B. If n is 2, the reaction is second order in B. If m or n is zero, the reaction is zero order in A or B, respectively, and the rate of the reaction is not affected by the concentration of that reactant. The overall reaction order is the sum of the orders with respect to each reactant. If m = 1 and n = 1, the overall order of the reaction is second order (m + n = 1 + 1 = 2).
Example 1
Writing Rate Laws from Reaction Orders
An experiment shows that the reaction of nitrogen dioxide with carbon monoxide:
NO2(g) + CO(g) → NO(g) + CO2(g)
is second order in NO2 and zero order in CO at 100 °C. What is the rate law for the reaction?
Solution
The reaction will have the form:
rate = k[NO2]m[CO]n
The reaction is second order in NO2; thus m = 2. The reaction is zero order in CO; thus n = 0. The rate law is:
rate = k[NO2]2[CO]0 = rate = k[NO2]2
Remember that a number raised to the zero power is equal to 1, thus [CO]0 = 1, which is why we can drop the concentration of CO from the rate equation: the rate of reaction is solely dependent on the concentration of NO2.
Check Your Learning
The rate law for the reaction:
H2(g) + 2 NO(g) → N2O(g) + H2O(g)
has been determined to be rate = k[NO]2[H2]. What are the orders with respect to each reactant, and what is the overall order of the reaction?
Answer:
order in NO = 2; order in H2 = 1; overall order = 3
Check Your Learning
In a transesterification reaction, a triglyceride reacts with an alcohol to form an ester and glycerol. Many students learn about the reaction between methanol (CH3OH) and ethyl acetate (CH3CH2OCOCH3) as a sample reaction before studying the chemical reactions that produce biodiesel:
CH3OH + CH3CH2OCOCH3 → CH3OCOCH3 + CH3CH2OH
The rate law for the reaction between methanol and ethyl acetate is, under certain conditions, determined to be:
rate = k[CH3OH]
What is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction?
Answer:
order in CH3OH = 1; order in CH3CH2OCOCH3 = 0; overall order = 1
Determining the Rate Law Using the Method of Initial Rates
It is sometimes helpful to use a more explicit algebraic method, often referred to as the method of initial rates, to determine the orders in rate laws. To use this method, we select two sets of rate data that differ in the concentration of only one reactant and set up a ratio of the two rates and the two rate laws. After canceling terms that are equal, we are left with an equation that contains only one unknown, the coefficient of the concentration that varies. We then solve this equation for the coefficient.
Example 2
Determining a Rate Law from Initial Rates
Ozone in the upper atmosphere is depleted when it reacts with nitrogen oxides. The rates of the reactions of nitrogen oxides with ozone are important factors in deciding how significant these reactions are in the formation of the ozone hole over Antarctica (Figure 1). One such reaction is the combination of nitric oxide, NO, with ozone, O3:
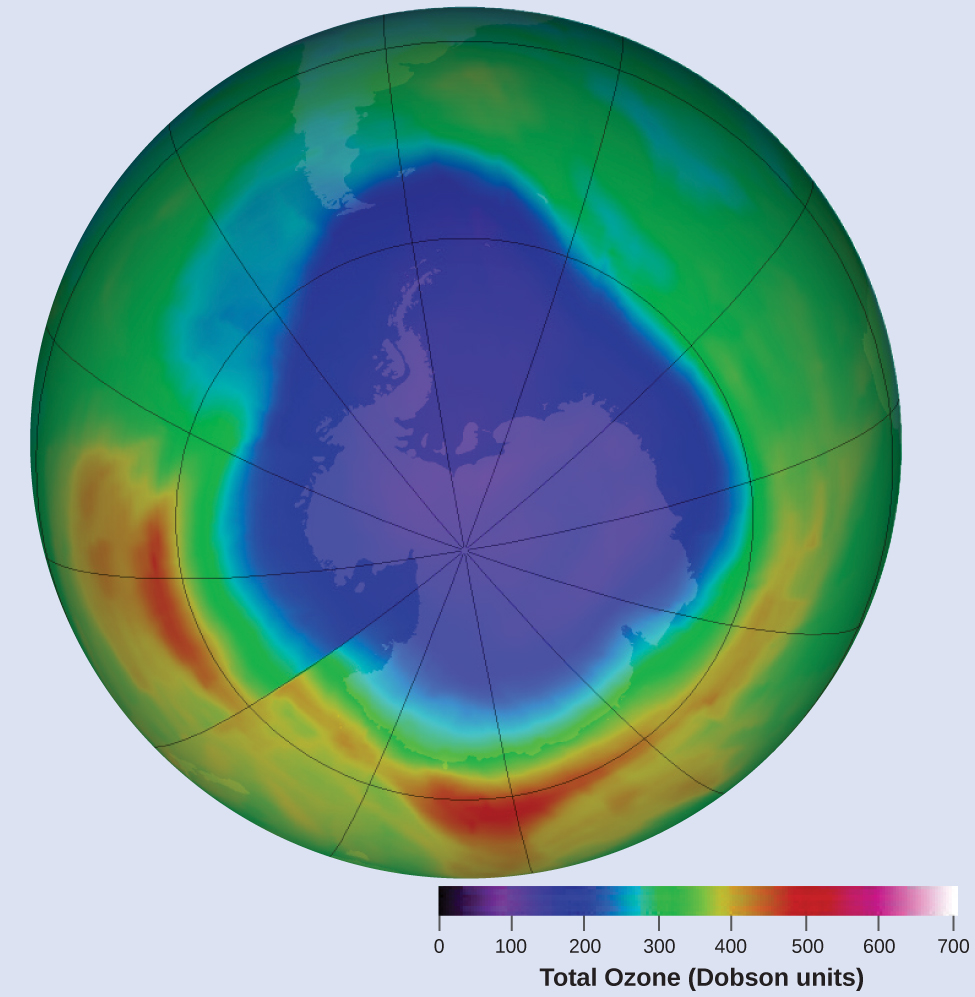
NO(g) + O3(g) → NO2(g) + O2(g)
This reaction has been studied in the laboratory, and the following rate data were determined at 25 °C.
| Trial | [NO] (M) | [O3] (M) | Initial Rate (M/s) |
| 1 | 1.00 × 10−6 | 3.00 × 10−6 | 6.60 × 10−5 |
| 2 | 1.00 × 10−6 | 6.00 × 10−6 | 1.32 × 10−4 |
| 3 | 1.00 × 10−6 | 9.00 × 10−6 | 1.98 × 10−4 |
| 4 | 2.00 × 10−6 | 9.00 × 10−6 | 3.96 × 10−4 |
| 5 | 3.00 × 10−6 | 9.00 × 10−6 | 5.94 × 10−4 |
Determine the rate law and the rate constant for the reaction at 25 °C.
Solution
The rate law will have the form:
rate = k[NO]m[O3]n
We can determine the values of m, n, and k from the experimental data using the following three-part process:
- Determine the value of m from the data in which [NO] varies and [O3] is constant. In the last three experiments, [NO] varies while [O3] remains constant. When [NO] doubles from trial 3 to 4, the rate doubles, and when [NO] triples from trial 3 to 5, the rate also triples. Thus, the rate is also directly proportional to [NO], and m in the rate law is equal to 1.
- Determine the value of n from data in which [O3] varies and [NO] is constant. In the first three experiments, [NO] is constant and [O3] varies. The reaction rate changes in direct proportion to the change in [O3]. When [O3] doubles from trial 1 to 2, the rate doubles; when [O3] triples from trial 1 to 3, the rate increases also triples. Thus, the rate is directly proportional to [O3], and n is equal to 1. The rate law is thus:
rate = k[NO][O3]
- Determine the value of k from one set of concentrations and the corresponding rate.
| k | = | |
| = | ||
| = | 2.20 × 107 M-1s-1 |
The large value of k tells us that this is a fast reaction that could play an important role in ozone depletion if [NO] is large enough.
Check Your Learning
Acetaldehyde decomposes when heated to yield methane and carbon monoxide according to the equation:
CH3CHO(g) → CH4(g) + CO(g)
Determine the rate law and the rate constant for the reaction from the following experimental data:
| Trial | [CH3CHO] (M) | Initial Rate (M/s) |
| 1 | 1.75 × 10−3 | 2.06 × 10−11 |
| 2 | 3.50 × 10−3 | 8.24 × 10−11 |
| 3 | 7.00 × 10−3 | 3.30 × 10−10 |
Answer:
rate = k[CH3CHO]2 with k = 6.73 × 10−6 M-1s-1
Example 3
Determining Rate Laws from Initial Rates
Using the initial rates method and the experimental data, determine the rate law and the value of the rate constant for this reaction:
2 NO(g) + Cl2(g) → 2 NOCl(g)
| Trial | [NO] (M) | [Cl2] (M) | Initial Rate (M/s) |
| 1 | 0.10 | 0.10 | 0.00300 |
| 2 | 0.10 | 0.15 | 0.00450 |
| 3 | 0.15 | 0.10 | 0.00675 |
Solution
The rate law for this reaction will have the form:
rate = k[NO]m[Cl2]n
As in Example 2, we can approach this problem in a stepwise fashion, determining the values of m and n from the experimental data and then using these values to determine the value of k. In this example, however, this time we will use a different approach to determine the values of m and n :
- Determine the value of m from the data in which [NO] varies and [Cl2] is constant. We can write the ratios with the subscripts x and y to indicate data from two different trials:
![Rendered by QuickLaTeX.com \dfrac{\text{rate}_{x}}{\text{rate}_{y}} = \dfrac{k[\text{NO}]_x^m[\text{Cl}_{2}]_x^n}{k[\text{NO}]_y^m[\text{Cl}_{2}]_y^n}](https://wisc.pb.unizin.org/app/uploads/quicklatex/quicklatex.com-28225e549d5a83efd668db9bbfbd04ba_l3.png)
Using the third trial and the first trial, in which [Cl2] does not vary, gives:
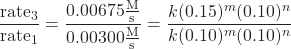
After canceling equivalent terms in the numerator and denominator, we are left with:
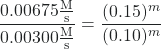
which simplifies to:
2.25 = (1.50)m
We can use natural logs to determine the value of the exponent m:
ln(2.25) = mln(1.50)
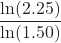 = m
= mm = 2
We can confirm the result, since:
1.502 = 2.25
- Determine the value of n from data in which [Cl2] varies and [NO] is constant.
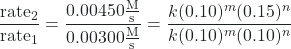
Cancelation gives:
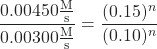
which simplifies to:
1.5 = (1.5)n
Thus n must be 1, and the form of the rate law is:
rate = k[NO]2[Cl2]
- Determine the numerical value of the rate constant k with appropriate units.
To determine the value of k once the rate law expression has been solved, simply plug in values from the first experimental trial and solve for k:
0.00300 M s-1 = k(0.10 M)2(0.10 M)
k = 3.0 M-2 s-1
Check Your Learning
Use the provided initial rate data to derive the rate law for the reaction whose equation is:
OCl–(aq) + I–(aq) → OI–(aq) + Cl–(aq)
| Trial | [OCl−] (M) | [I−] (M) | Initial Rate (M/s) |
| 1 | 0.0040 | 0.0020 | 0.00184 |
| 2 | 0.0020 | 0.0040 | 0.00092 |
| 3 | 0.0020 | 0.0020 | 0.00046 |
Determine the rate law expression and the value of the rate constant k with appropriate units for this reaction.
Answer:
rate = k[OCl–]2[I–]
k = 5.75 x 104 M-2 s-1
Reaction Order and Rate Constant Units
Reaction orders also play a role in determining the units for the rate constant k. In Example 2, a second-order reaction, we found the units for k to be M-1s-1, whereas in Example 3, a third order reaction, we found the units for k to be M−2s-1. The following summarizes the rate constant units for common reaction orders:
| Reaction Order | Units of k |
| zero | M s-1 |
| first | s−1 |
| second | M-1 s-1 |
| third | M-2 s−1 |
| m+n | M1-(m+n) s-1 |
Note that the units in the table can also be expressed in terms of mol/L instead of molarity (M). Also, units of atoms, molecules, atm, or units of time other than the second (such as minutes, hours, days) may be used, depending on the situation.
Key Concepts and Summary
An extremely useful way to determine the rate of reaction is by using a rate law, which contains a rate constant and the concentration of all reactants to a reaction order. The reaction order must be determined experimentally, with one way being the method of initial rates. The method of initial rates looks at how the initial rate of a reaction changes when the initial concentration of the reactants are also changed, one reactant at a time. Once the orders of the reaction are known, the rate constant can also be calculated.
Key Equations
- rate = k[A]m[B]n
Glossary
- method of initial rates
- an algebraic method that can be used to determine the rate law of a reaction
- rate constant
- defined as k, which is specific for a particular reaction at a particular temperature
- rate law (or rate equation)
- a mathematical expression that describes the relationship between the rate of a chemical reaction and the concentration of its reactants
- reaction order
- the exponents in a rate law that describe the effect of a reactant concentration on the reaction rate. Adding together all reaction orders gives the overall reaction order.
Chemistry End of Section Exercises
- Doubling the concentration of a reactant increases the rate of a reaction four times. What is the order of the reaction with respect to that reactant?
- Tripling the concentration of a reactant increases the rate of a reaction three times. What is the order of the reaction with respect to that reactant?
- How will each of the following affect the rate of the reaction if:
CO(g) + NO2(g) → CO2(g) + NO(g) rate = k[NO2]2
- Decreasing the concentration of NO2 from 0.50 M to 0.250 M, while keeping the CO concentration unchanged.
- Increasing the concentration of CO from 0.010 M to 0.030 M while keeping the NO2 concentration unchanged.
- Regular flights of supersonic aircraft in the stratosphere are of concern because such aircraft produce nitric oxide, NO, as a byproduct in the exhaust of their engines. Nitric oxide reacts with ozone, and it has been suggested that this could contribute to depletion of the ozone layer. The reaction NO(g) + O3(g) → NO2(g) + O2(g) is first order with respect to both NO and O3 with a rate constant of 2.20 × 107 M-1s-1. What is the instantaneous rate of disappearance of NO when [NO] = 3.3 × 10−6 M and [O3] = 5.9 × 10−7 M?
- Alcohol is removed from the bloodstream by a series of metabolic reactions. The first reaction produces acetaldehyde; then other products are formed. The following data have been determined for the rate at which alcohol is removed from the blood of an average male, although individual rates can vary by 25–30%.
Trial 1 Trial 2 Trial 3 [C2H5OH] 4.4 × 10−2 3.3 × 10−2 2.2 × 10−2 Rate (M/s) 2.0 × 10−2 2.0 × 10−2 2.0 × 10−2 Determine the rate equation, the rate constant, and the overall order for this reaction.
- Nitrogen(II) oxide reacts with chlorine according to the equation:
2 NO(g) + Cl2(g) → 2 NOCl(g)
The following initial rates of reaction have been observed for certain reactant concentrations:
[NO] (M) [Cl2] (M) Rate (M/hr) 0.50 0.50 1.14 1.00 0.50 4.56 1.00 1.00 9.12 What is the rate equation that describes the rate’s dependence on the concentrations of NO and Cl2? What is the rate constant? What are the orders with respect to each reactant?
- Consider the experimental data in the table below regarding the reaction:
2 NO (g) + Cl2 (g) → 2 NOCl (g)
Experiment [NO]0 [Cl2]0 Initial Rate (M/s) 1 0.020 M 0.010 M 8.00 × 10−5 2 0.020 M 0.020 M 1.60 × 10−4 3 0.020 M 0.040 M 3.20 × 10−4 4 0.040 M 0.080 M 2.56 ×10−3 - Based on the tabulated data, what is the rate law for the overall reaction?
- What is the value of the rate constant, k, for this reaction?
Answers to Chemistry End of Section Exercises
- second-order
- first-order
- (a) Decreases the rate by a factor of 4
(b) Since CO does not appear in the rate law, the rate is not affected. - 4.3 × 10−5 M s-1
- rate = k; k = 2.0 × 10−2 M-1 hr-1; The reaction is zero order.
- rate = k[NO]2[Cl2]; k = 9.12 M−2 hr−1; second order in NO; first order in Cl2
- (a) Rate = k[NO]2[Cl2]
(b) 2.0 × 101 M-2 s-1Left-click here to watch Exercise 7 problem solving video.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

