D28.1 Buffer Solutions
A mixture containing a weak acid and its conjugate base, such as acetic acid and sodium acetate [CH3COOH(aq) + CH3COONa(aq)], or a mixture containing a weak base and its conjugate acid, such as ammonia and ammonium chloride [NH3(aq) + NH4Cl(aq)], is a buffer solution. A buffer solution resists changes in pH when small amounts of a strong acid or a strong base are added.

A solution made from equal concentrations of CH3COOH and CH3COO– is slightly acidic because Ka,acetic acid > Kb, acetate anion. When a strong base, such as NaOH, is added to this solution, the OH– anions react with the H3O+ cations present in the solution in a very product-favored reaction, decreasing concentrations of H3O+:
In response to this decrease in [H3O+] upon the addition of NaOH, the Ka,acetic acid equilibrium reaction:
shifts towards producing more products, thereby restoring [H3O+] to almost the value it was before NaOH was added. Therefore, the net effect of the added NaOH is to convert some of the CH3COOH to CH3COO−. In other words, if you add the two above reaction equations, you would have:
Overall, there is minimal change in the solution’s H3O+ concentration. (Note that the added NaOH may react directly with H3O+ or CH3COOH, because both species are present in the buffer solution when NaOH is added. Regardless of which acidic species NaOH is reacting with directly, the final result is the same, and there is minimal change in the solution’s pH.)
Similarly, when a strong acid, such as HCl, is added, the net effect of the added H3O+ is to convert some of the CH3COO− to CH3COOH:
And again, there is only a minimal change in the solution’s pH.
These concepts are illustrated in the figure below. When a small amount of a strong acid or strong base is added, a buffer solution can moderate changes to pH because it consists of a weak acid that can react with added strong base as well as a weak base that can react with added strong acid.
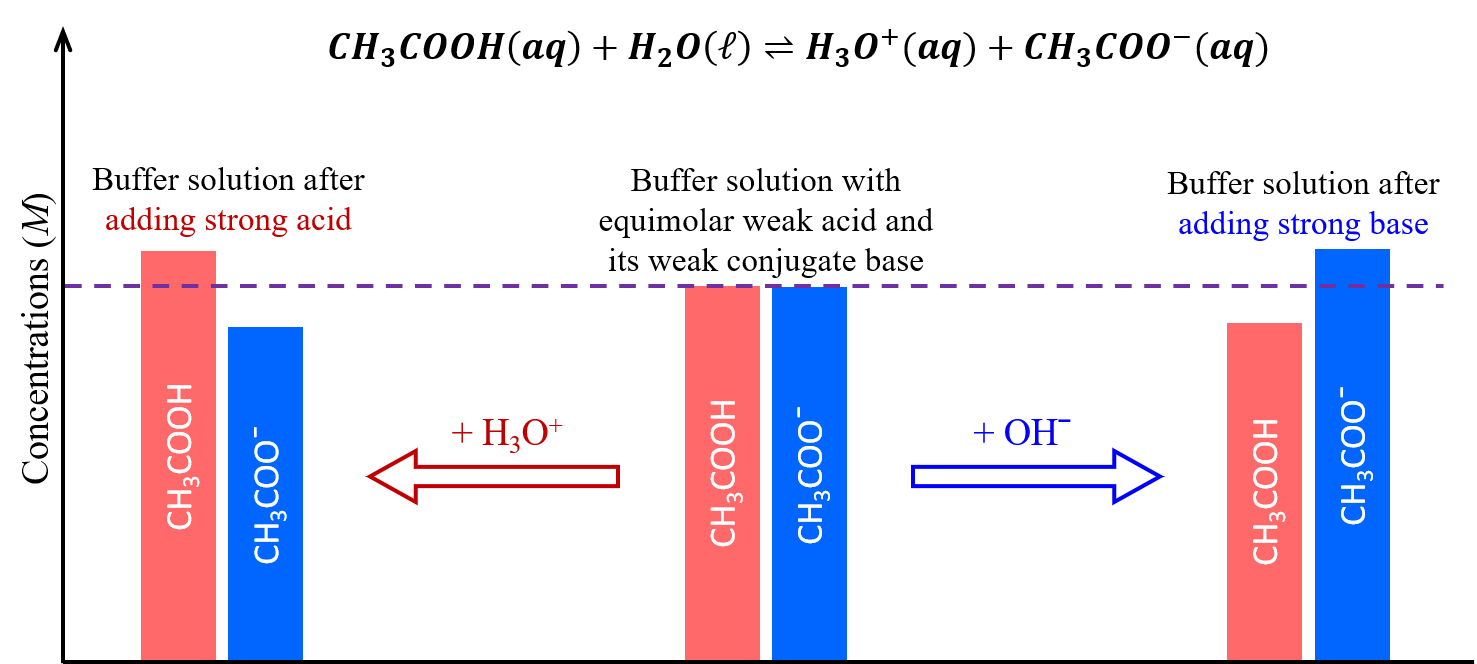
The weak base and weak acid in a buffer solution are typically a conjugate acid-base pair, both serving to maintain a single dynamic equilibrium that responds to additions of other acids and bases. If they are not a conjugate acid-base pair, then there would be two dynamic equilibria at play, which significantly complicates the buffering actions.
Activity: pH of a Buffer Solution
Exercise: Characteristics of Buffer Solutions
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

