D13.5 Ethers
An ether functional group contains the group –O–, which bonds to two different R groups and is found in the middle of a molecule.
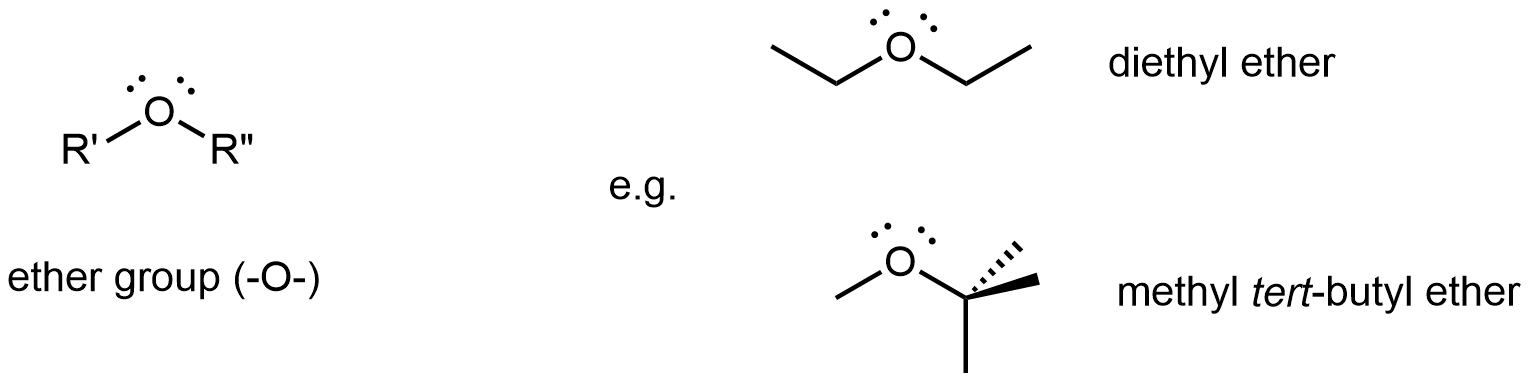
Diethyl ether, the most widely used ether, is a colorless, volatile liquid that is highly flammable. It was first used in 1846 as an anesthetic, but better anesthetics have now largely taken its place. Diethyl ether and other ethers are now used primarily as solvents for gums, fats, waxes, and resins. Methyl tert-butyl ether (abbreviated MTBE) is used as an additive for gasoline. MTBE belongs to a group of chemicals known as oxygenates due to their capacity to increase the oxygen content of gasoline.
Exercise: Ether Hybridization and Local Bond Geometry
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

