D7.6 Exceptions to the Octet Rule
Some covalent molecules contain one or more atoms that do not have an octet. Such molecules fall into three categories.
Odd-electron Molecules
A molecule that contains an odd number of electrons must have at least one electron unpaired and therefore must have an atom with fewer than eight electrons in its valence shell (typically it’s seven electrons). A molecule with at least one unpaired electron is called a free radical. Nitric oxide, NO, which is produced in internal combustion engines when oxygen and nitrogen react at high temperatures, is an example.
To draw the Lewis structure for an odd-electron molecule, follow the same steps as outlined previously, but recognize that an odd-electron molecule must have less than an octet on some atom. For example, the Lewis structure for NO is:
![]()
Note that trying to drawing a triple bond for NO would cause one of the atoms to exceed an octet, which is impossible for a second-period atom.
Molecules with an Incomplete Octet on a Central Atom
Some molecules contain a central atom that does not have a filled valence shell. Usually, these central atoms are from groups 2 (IIA) and 13 (IIIA). For example, the Lewis structure of beryllium chloride, BeCl2, shows beryllium with only four electrons, and that of boron trifluoride, BF3, shows boron with only six electrons.
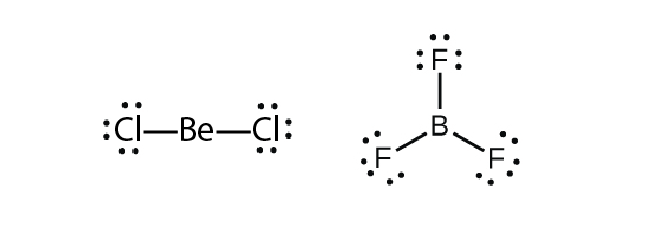
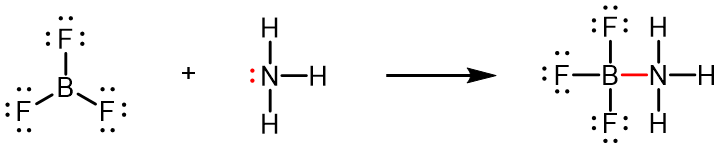
It is possible to draw a structure for each of these molecules where there are double bonds to the central atom and therefore an octet. For example, BF3 with one B=F double bond would satisfy the octet rule. However, such a Lewis structure would have a negative formal charge on B and a positive formal charge on F (boron is much less electronegative than fluorine). Moreover, experimental evidence tells us that the bond lengths in BF3 are closer to B–F single bonds. The observed reactivity of BF3 is also consistent with less than an octet on boron: BF3 reacts readily with NH3, with the lone pair on nitrogen (red) forming a bond (red) that completes the octet on the boron atom:
Molecules with More Than an Octet on a Central Atom
Some Lewis structures show more than four pairs of electrons around the central atom. This usually happens when the central atom is in the third or a higher period (n ≥ 3). Molecules with more than an octet around a central atom are called hypervalent molecules. For example, in the Lewis structure for PCl5, the central phosphorus atom is surrounded by five pairs of electrons. In SF6, sulfur has six pairs of electrons.
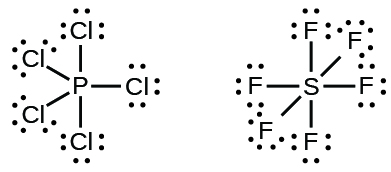
Exercise: Exceptions to the Octet Rule
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

