D1.6 Structure, Energy, and States of Matter
Consider changes of state from solid to liquid to gas. The idea that atomic-scale particles attract one another and adopt a low-energy arrangement unless energy is supplied from an external source can be applied to such changes.
The kinetic-molecular theory states that atomic-level particles are in constant, random motion. How fast the particles move depends on temperature. As temperature increases the average speed of the particles increases; hence their kinetic energies also increase. The average of the energies of the particles is proportional to the absolute temperature (in units of kelvins). Near 0 K the particles have very little kinetic energy and adopt a structure with minimum potential energy.
Temperature and motion of particles affect whether a substance is a solid, liquid, or gas. View this simulation of the behavior of noble gas atoms in solids, liquids, and gases. Choose “States”, then choose “Neon” (upper right corner). Click on each of the boxes: “Solid”, “Liquid”, and “Gas” (or use “Heat” (below the container of molecules) to raise the temperature).
Based on the simulation, write in your notebook a description of the differences in position and movement of the molecules in solids, liquids, and gases.
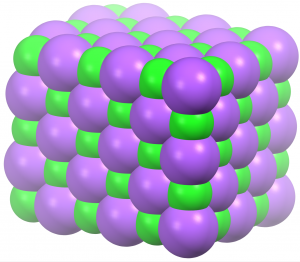
Let’s apply these ideas to sodium chloride, which is a solid at low temperature. Sodium ions and chloride ions are packed closely together in a regular pattern, as seen in the diagram at the right. This arrangement minimizes the potential energy by bringing oppositely charged particles closer together. A little above 0 K the ions are in motion: each vibrates a bit around its specific location, but no ion has sufficient kinetic energy to overcome the Coulombic attractions that hold it in place. Thus, no ion exchanges place with any other ion. This atomic-scale structure is consistent with macroscopic properties: a solid is rigid because the regular pattern of its structure does not change.
As temperature increases, the average kinetic energy of the ions increases: the ions move more, vibrating farther from their average positions. On the macroscopic scale, the solid expands because each vibrating ion pushes against neighboring ions. Pushing neighboring ions away enlarges the space occupied by each ion and the crystal’s volume increases. Eventually, the vibrations are large enough that ions can move relative to each other and the regular arrangement becomes much more random: the solid melts. Ions are still close together but they can move past one another, so the liquid has no specific shape. The liquid is fluid and can be poured. For sodium chloride, because the potential energy is lowered significantly by Coulombic attractions among the ions, the temperature required for melting is high: 1074 K (801 °C).
Further increase in temperature makes the motion of the atoms faster and at 1738 K (1465 °C) the liquid sodium chloride boils, forming a gas. The sodium ions and chloride ions move much further apart, which means they bump into each other much less often. The gas is fluid but expands to fill whatever volume is available and has much lower density than the liquid or solid.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

