D15.3 Polymer Structure and Properties
In polyethylene, the addition reactions convert all C=C double bonds in the monomers to C-C single bonds in the polymer, and join the monomers without losing any atoms. Hence, this polymer at a molecular level consists of a collection of long-chain alkane molecules, most of which contain tens of thousands of carbon atoms. Many of polyethylene’s properties are what we would expect from this molecular composition.
For example, because polyethylene is a mixture of long alkane molecules, each slightly different chain length, it melts (softens) over a range of temperatures rather than having a single melting point. You may have noticed that some plastics, when wrapped around something in a microwave oven, soften and change shape but never become liquid.
In general, if the intermolecular forces between the polymer strands are smaller, so that it is easier for them to move past one another, the polymer will be softer and easier to scratch. If the intermolecular forces between the strands are sufficiently strong, the polymer will be harder or more rigid.
This leads to an important idea in the field of materials science: materials can be “tuned” to give exactly the properties desired. By adjusting the strengths of the intermolecular forces one can obtain plastic materials with a range of properties.
For polyethylene, the extent of branching of a polymer strand can be varied. Depending on the production process, a polymer strand can be a very long linear chain or there can be branching in the chain:
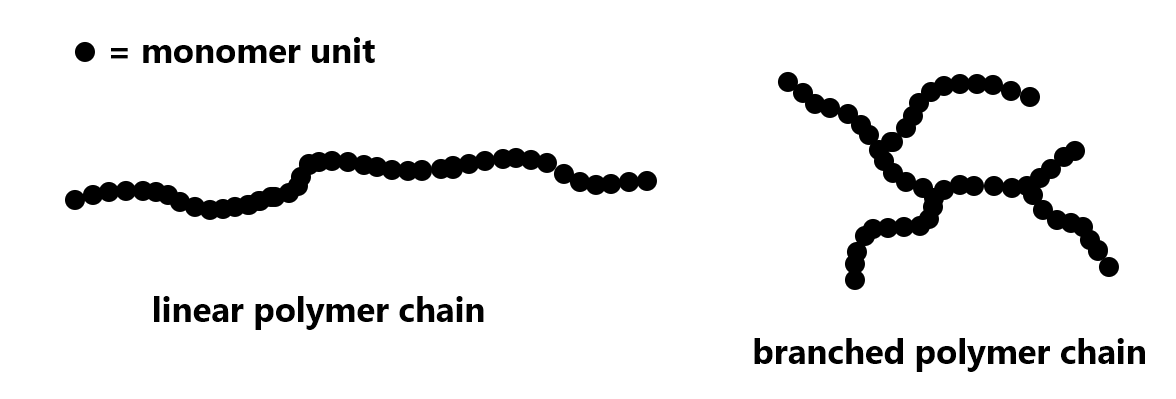
Exercise: Polymer Structure and Properties
The strength of LDFs falls off as a function of 1/R6, where R is the distance between molecules. So the closer two molecules are to each other, the stronger the LDFs. For polymers, their density can be used to estimate how closely packed the polymer strands are to each other.
Polymer properties can also be affected by cross linking. A cross-link is a covalent σ bond between two separate polymer strands that is not at the end of either strand.
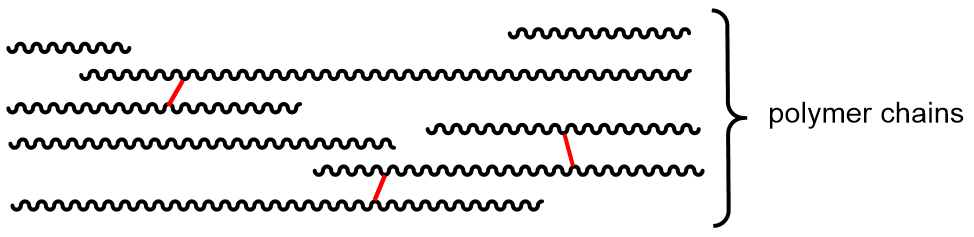
Cross links increase the molecular weight and limit the motions of the polymer strands with respect to one another. With enough cross links, a sample of a polymer can become a three-dimensional network held by σ bonds—a single gigantic molecule. Another way to think about cross links: because they are covalent bonds, they are typically stronger than intermolecular forces between polymer chains and therefore amplify the effect of increasing intermolecular forces on polymer properties.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

