D14.1 Alcohols
An alcohol functional group contains a hydroxyl (–OH) group covalently bonded to a carbon atom. (Note that the –OH group is covalently bonded; the OH is not a hydroxide ion.) The names of alcohols are derived from the names of the corresponding alkanes by removing the final “e” and adding “ol”. For example, the structure of the simplest alcohol is derived from the structure of methane by replacing one H atom with an OH group (CH3OH). It is named methanol.
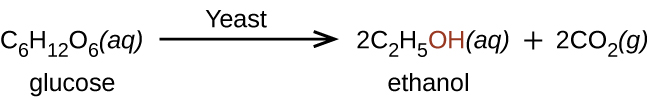
Ethanol is produced by some species of yeast that catalyze fermentation of various sugars:
Ethanol can also be synthesized using the addition reaction of ethene and water using an acid as a catalyst:
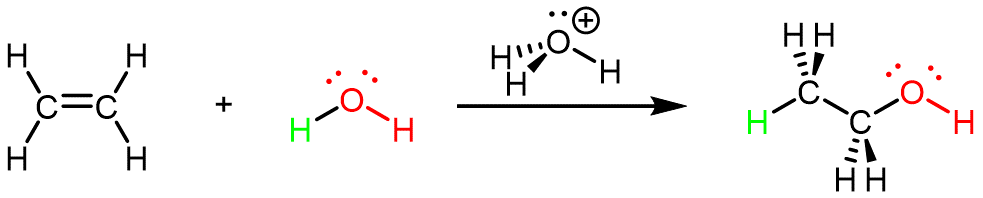
This reaction is called an addition reaction because all atoms contained in the reactant molecules are contained in (added to) the product molecule. (In the reaction above, the two C atoms and four H atoms in ethene as well as both H atoms and the O atom in water are in the CH3CH2OH product molecule.)
Exercise: Structures of Alcohols
Knowing how to systematically name compounds can be helpful, especially when trying to decipher whether the line structures shown are constitutional isomers or not. See the nomenclature appendix for additional descriptions on how to name alcohol compounds. (You will not be explicitly tested on nomenclature in this course.)
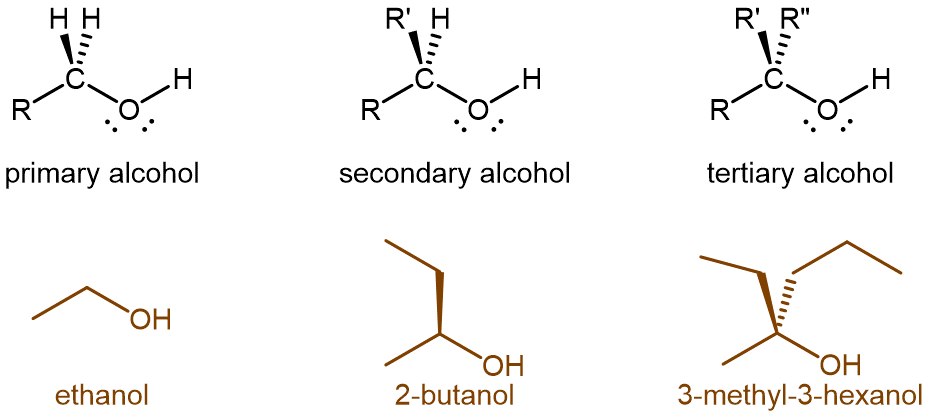
Alcohol molecules can be classified according to the number of alkyl groups attached to the carbon with the -OH group, as shown in the figure below. If one alkyl group (or only hydrogen) is attached to that carbon, the alcohol is a primary (1º) alcohol. If two alkyl groups are attached, the alcohol is a secondary (2º) alcohol. If three alkyl groups are attached, the alcohol is a tertiary (3º) alcohol.
Exercise: Identifying Alcohols from Structure
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

