D40.1 Voltaic Cell Potential
When a voltaic cell is connected to a load, such as a light bulb, an electric current flows because there is a difference in electrical potential between the two electrodes. That electrical potential difference can be measured using a potentiometer (a voltmeter).
The voltaic cell shown below involves the spontaneous reaction:
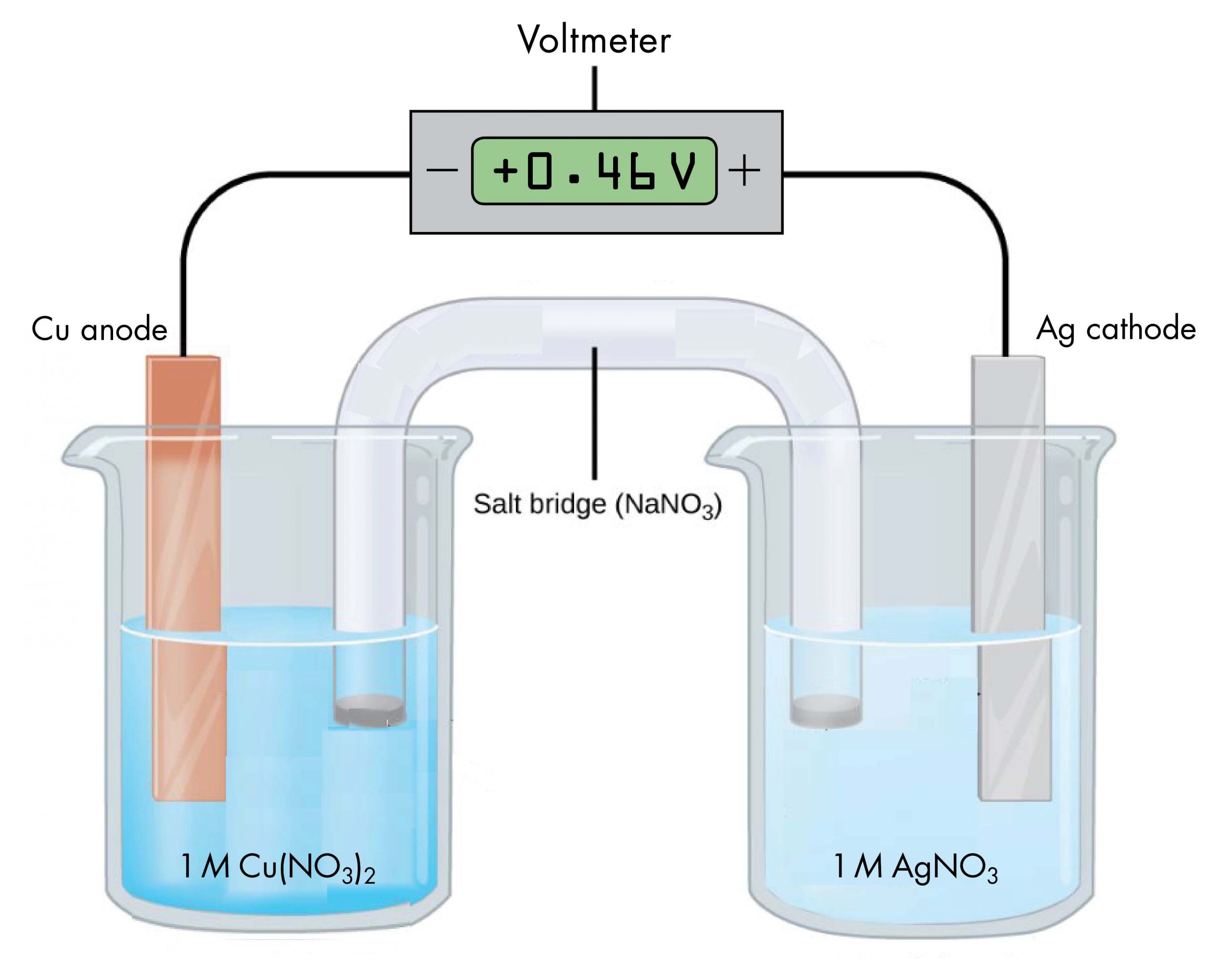
According to the reaction equation, copper loses electrons and is oxidized to copper(II) ions, so the half-cell with the copper electrode is the anode. According to the reaction equation, silver ions gain electrons and are reduced to silver, so the half-cell with the silver electrode is the cathode. The copper electrode has a more negative potential than the silver electrode.
When the more negative copper electrode is connected to the negative terminal of the voltmeter and the more positive silver electrode is connected to the positive terminal of the voltmeter, the meter reads +0.46 V. This reading is called the cell potential, Ecell. It is a measure of the energy per unit charge available from a redox reaction (V = J/C). A positive cell potential indicates how much electrical work a spontaneous reaction in a voltaic cell can do per unit electric charge moving though the circuit.
Under standard-state conditions (1 bar or 1 M), the cell potential is the standard cell potential, E°cell (pronounced “E-standard-cell”). Thus, based on the voltmeter reading in the figure above, E°cell = 0.46 V.
A voltmeter measures the difference in electrical potential between its positive terminal and its negative terminal. Because the positive meter terminal is on the right in the figure above, the cell potential is the difference in electrical potential between the right-hand half-cell and the left-hand half-cell, and we can write:
In the above figure, all concentrations are 1 M (standard-state conditions), so we can also write,
If the wire connections are reversed, a typical voltmeter would read −0.46 V. This provides an experimental way to determine which half-cell is the cathode and which is the anode: a positive voltmeter reading indicates that the meter’s negative terminal is connected to the anode and the positive terminal is connected to the cathode; a negative voltmeter reading indicates the negative terminal is connected to the cathode and the positive terminal is connected to the anode.
The cell potential of a voltaic cell depends on the substances in each half-cell and the concentrations of solutions and partial pressures of gases involved in the half-cell. A salt bridge must be present to complete an electric circuit. In the salt bridge when the cell generates electric current, anions move toward the anode and cations move toward the cathode.
Exercise: Voltaic Cell Terminology
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

