D13.3 Functional Groups
There are more than twenty million known organic compounds, so it would be impossible to memorize chemical properties for each one. Fortunately, we can make use of functional groups to deduce the likely chemical and physical properties of a molecule. A functional group is specific structure that has similar chemical properties whenever it is present in a molecule. Even if other parts of a molecule are quite different, a specific functional group usually reacts the same way.
Because C–C and C–H bonds are strong, alkanes are unreactive at room temperature; they are used primarily as fuels (Section: Hydrocarbons). The alkane parts of molecules usually don’t participate in reactions and are not defined as functional groups. An alkyl group is a portion of an alkane molecule bonded to something else. Examples of alkyl groups include -CH3 (methyl), -CH3CH2 (ethyl), and -CH(CH3)2 (2-propyl). (See alkane nomenclature for more examples.) We often use R (for the Rest of the molecule) to designate any alkyl group (or sometimes another type of group) in a molecule. When there are two or more different alkyl groups, we use R, R’, R”, etc. For example, R and R’, are trans to each other in the alkene structure below:
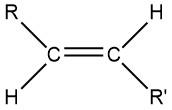
When a molecule is drawn using R or R’ for alkyl groups, greater focus is put on a specific functional group, in this case, the alkene C=C bond.
Sections Alkenes and Alkynes described the functional groups in alkenes and alkynes. The aromatic functional group was discussed in Section: Aromatic Molecules. The next few sections consider functional groups that contain heteroatoms (atoms other than carbon and hydrogen). Each of these functional groups has its own specific chemical reactivity. Each functional group can also affect the types of intermolecular forces present, giving rise to differing physical properties.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

