D13.4 Aldehydes and Ketones
An aldehyde or a ketone contains a carbonyl group, a carbon atom double bonded to an oxygen atom. The carbon atom in a carbonyl group is called the carbonyl carbon. In an aldehyde functional group, the carbonyl carbon is also bonded to a hydrogen atom. Hence, an aldehyde group can at most bond to one R group, and the aldehyde group is always at the end of a chain of carbon atoms.
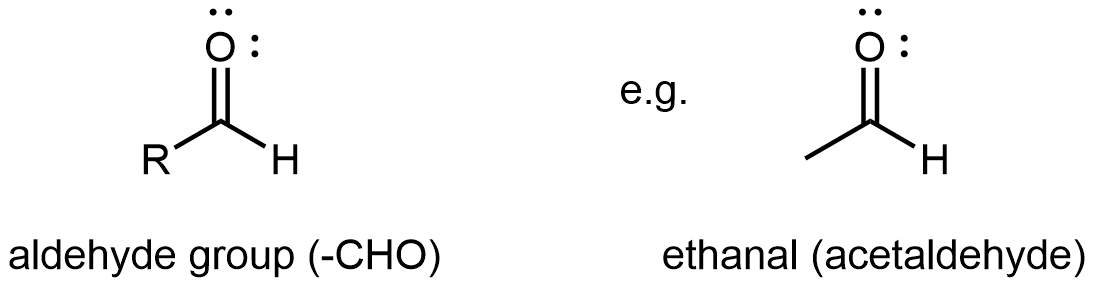
A ketone functional group consists solely of the carbonyl group. It bonds to two R groups, which may be the same or different, and is found partway along a chain of carbon atoms.
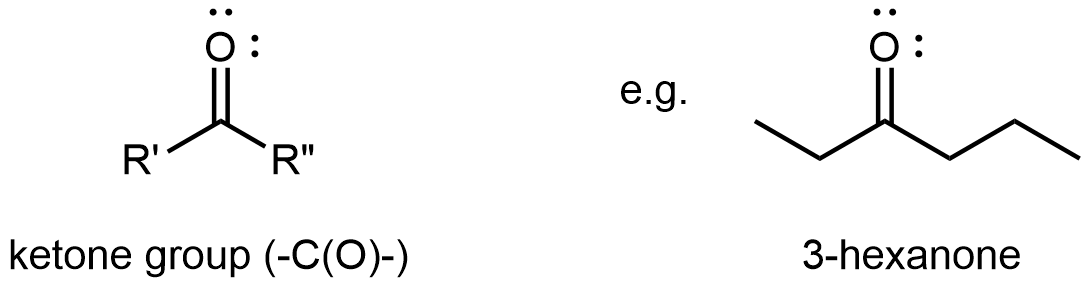
The reactivity of both aldehydes and ketones are directly related to the reactivity of the carbonyl group.
Formaldehyde, the simplest aldehyde, is a colorless gas with a pungent and irritating odor. It is sold in an aqueous solution called formalin, which contains about 37% formaldehyde by mass.
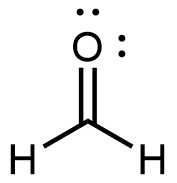
Formaldehyde causes coagulation of proteins, so it kills bacteria (and any other living organism) and stops many of the biological processes that cause tissues to decay. Thus, formaldehyde is used for preserving tissue specimens and embalming bodies. It can also be used to sterilize soil or other materials. Formaldehyde is used in the manufacture of Bakelite, a hard plastic having high chemical and electrical resistance.
Dimethyl ketone, CH3COCH3, commonly called acetone, is the simplest ketone. It is a colorless liquid that can be made commercially by fermenting corn or molasses. Among its many uses are: as a solvent for lacquer (including fingernail polish), cellulose acetate, cellulose nitrate, acetylene, plastics, and varnishes; as a paint and varnish remover; and as a solvent in the manufacture of pharmaceuticals and chemicals.
Exercise: Ketone Hybridization and Local Bond Geometry
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

