D15.2 Addition Polymers
Addition polymers are made by addition reactions, where two molecules combine to form a single product molecule (we have already seen examples of addition reactions involving alkenes). Typical monomers for addition polymerization have at least one C=C double bond.
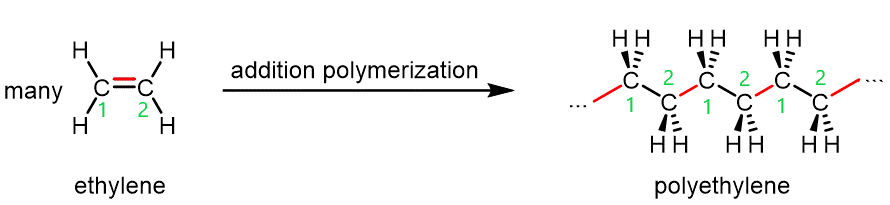
The figure below shows polyethylene, an addition polymer, forming from ethylene (ethene, H2C=CH2) monomers.
The addition polymerization reaction can be initiated by a molecule with an unpaired electron—a free radical. An example initiator is an organic peroxide, which can form two free radicals when the relatively weak O-O bond breaks:
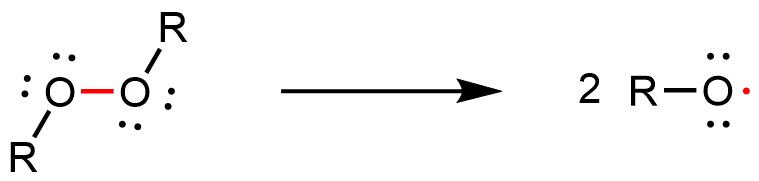
Activity: Analyzing Peroxide Decomposition
When a radical encounters an ethylene monomer, the ethylene π bond breaks. One electron from the π bond goes to pair with the electron from the radical and form a σ bond. The other electron from the π bond remains a radical, and can go on to react with another ethylene monomer.
The process illustrated in the figure above repeats with many, many more monomers, sometimes as many as 100,000 units, yielding a long polymer chain of carbon atoms.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

