D31.1 Rate Constant
Different substances are likely to react at different rates. Moreover, for most reactions, the rate of the reaction is dependent on one or more reactant concentrations:
Here, k is the rate constant, a proportionality constant independent of reactant concentrations that is specific to a particular reaction at a particular temperature. (We will revisit this equation later and discuss the implication of the exponents.) Because k is independent of reactant concentrations, it is a useful value when comparing rates of different reactions.
Just like the equilibrium constant, the rate “constant” is only constant at a given temperature. (Note that the equilibrium constant is a upper-case “K“, while the rate constant is a lower-case “k“.) The value of the rate constant is determined by three main factors, the first two of which depend on temperature:
- To react, molecules must come close enough to each other to exchange energy and perhaps to break and form bonds; that is, molecules must collide. The rate constant is proportional to the rate of collisions:
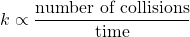
- For a reaction to occur, there must be sufficient energy in the reactant molecule or molecules to allow electrons to rearrange to break and/or form bonds. If all else are equal, a smaller energy requirement leads to a larger rate constant.
- The reacting molecules must collide in an orientation that allows the reaction to proceed; that is, even if the molecules have enough energy, a collision is more likely to result in reaction when the molecules are oriented in certain ways with respect to each other.
Let’s consider these factors in more detail from a molecular perspective.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

