D8.6 Petroleum Chemistry
Petroleum (from Latin, petra: “rock”, oleum: “oil”) consists primarily of naturally occurring hydrocarbons, predominantly alkanes and cycloalkanes. The alkane chains can be quite long, and properties such as melting point and boiling point usually vary smoothly and predictably as a function of the number electrons in various linear alkane molecules.
| Alkane | Molecular Formula | Number of Electrons | Melting Point (°C) | Boiling Point (°C) | Phase at Room Temperature |
|---|---|---|---|---|---|
| methane | CH4 | 10 | –182.5 | –161.5 | gas |
| ethane | C2H6 | 18 | –183.3 | –88.6 | gas |
| propane | C3H8 | 26 | –187.7 | –42.1 | gas |
| butane | C4H10 | 34 | –138.3 | –0.5 | gas |
| pentane | C5H12 | 42 | –129.7 | 36.1 | liquid |
| hexane | C6H14 | 50 | –95.3 | 68.7 | liquid |
| heptane | C7H16 | 58 | –90.6 | 98.4 | liquid |
| octane | C8H18 | 66 | –56.8 | 125.7 | liquid |
| nonane | C9H20 | 74 | –53.6 | 150.8 | liquid |
| decane | C10H22 | 82 | –29.7 | 174.0 | liquid |
| tetradecane | C14H30 | 114 | 5.9 | 253.5 | solid |
| octadecane | C18H38 | 146 | 28.2 | 316.1 | solid |
| Table: Melting and boiling points of alkanes. | |||||
Petroleum is the main source of hydrocarbon fuels, such as LP gas, gasoline, and fuel oil. These are separated by fractional distillation, a process in which petroleum is boiled and the different hydrocarbons condense to liquids at different temperatures (Figure below). Fractional distillation takes advantage of the differences in boiling-point of the various component substances. The different boiling points arise from the differences in the London dispersion forces between molecules.
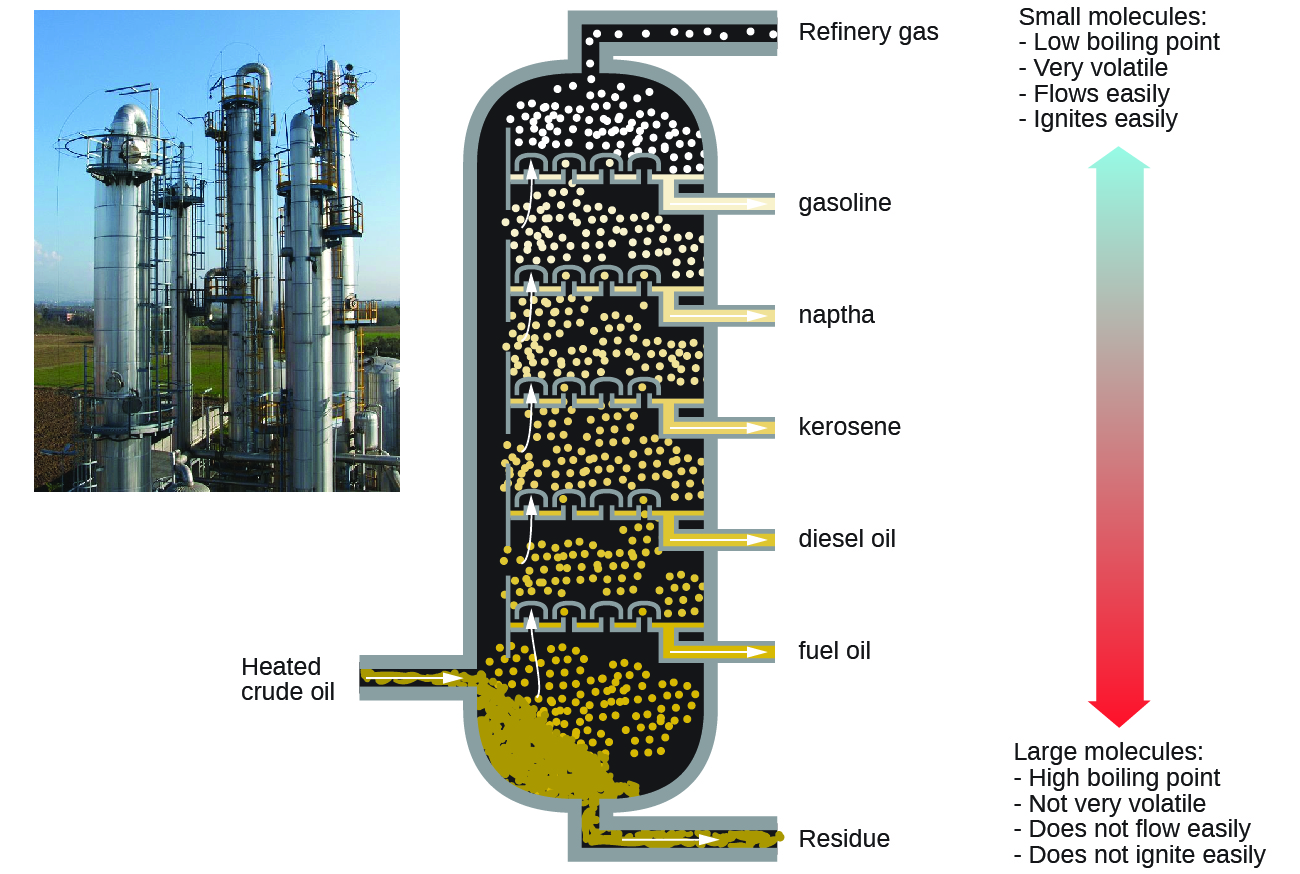
Gasoline is a liquid mixture of linear and branched alkanes, each containing five to twelve carbon atoms. Gasoline often contain various additives to improve its performance as a fuel. Kerosene, diesel fuel, motor oil, and fuel oil are primarily mixtures of alkanes made of larger molecules with more electrons than gasoline molecules.
Provided there is plenty of oxygen available, combustion converts almost all the carbon in the alkane fuel to carbon dioxide and water. Thus combustion of alkanes invariably adds water vapor and CO2 to the atmosphere—a human contribution to global warming.
Because there is greater demand for gasoline than for other components of petroleum, catalytic cracking is used in petroleum refining to break up larger molecules into smaller molecules, some of which are within the gasoline range of 5–12 carbon atoms. Catalytic cracking involves temperatures of 480–550 °C and a catalyst—conditions that can break (crack) carbon-carbon bonds and rearrange molecular structures. The hydrocarbon molecules are broken up in a fairly random way to produce mixtures of smaller molecules, some of which have carbon-carbon double bonds. One possible reaction involving C15H32 might be:
The alkene products, ethene and propene, are important for producing other organic chemicals. The octane product is a component of gasoline. Note that catalytic cracking involves temperatures higher than fractional distillation as well as a catalyst in order to break carbon-carbon bonds (as opposed to overcoming LDFs between hydrocarbon molecules).
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

