D16.4 DNA and Lipids
DNA
Deoxyribonucleic acid (DNA) consists of two polymer strands that coil around each other, forming a double helix. The monomer units of DNA strands are nucleotides, each consists of a phosphate group and a nucleobase attached to a sugar (deoxyribose).
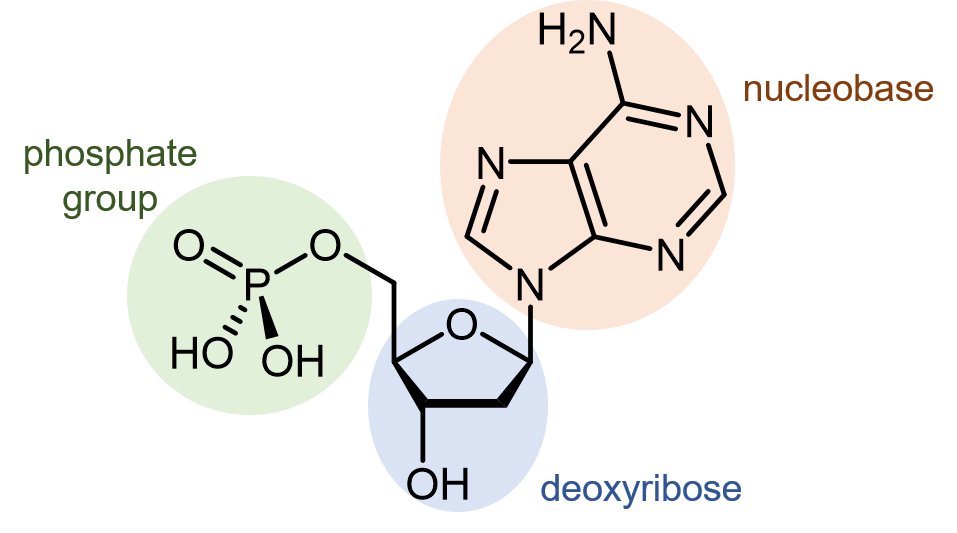
There are four different nucleobases found in DNA: adenine (abbreviated A), thymine (T), guanine (G) and cytosine (C).
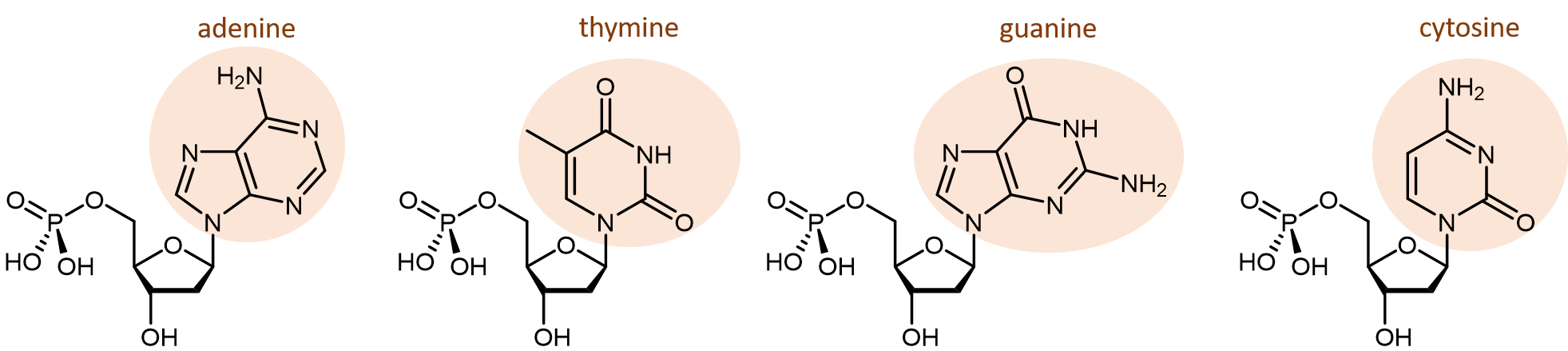
The polymerization reactions forming a DNA strand are also condensation reactions, which form phosphodiester bonds joining the nucleotides. Each DNA polymer strand has a backbone of alternating sugar group and phosphodiester bond, with nucleobases forming side-chains bonded to the backbone.
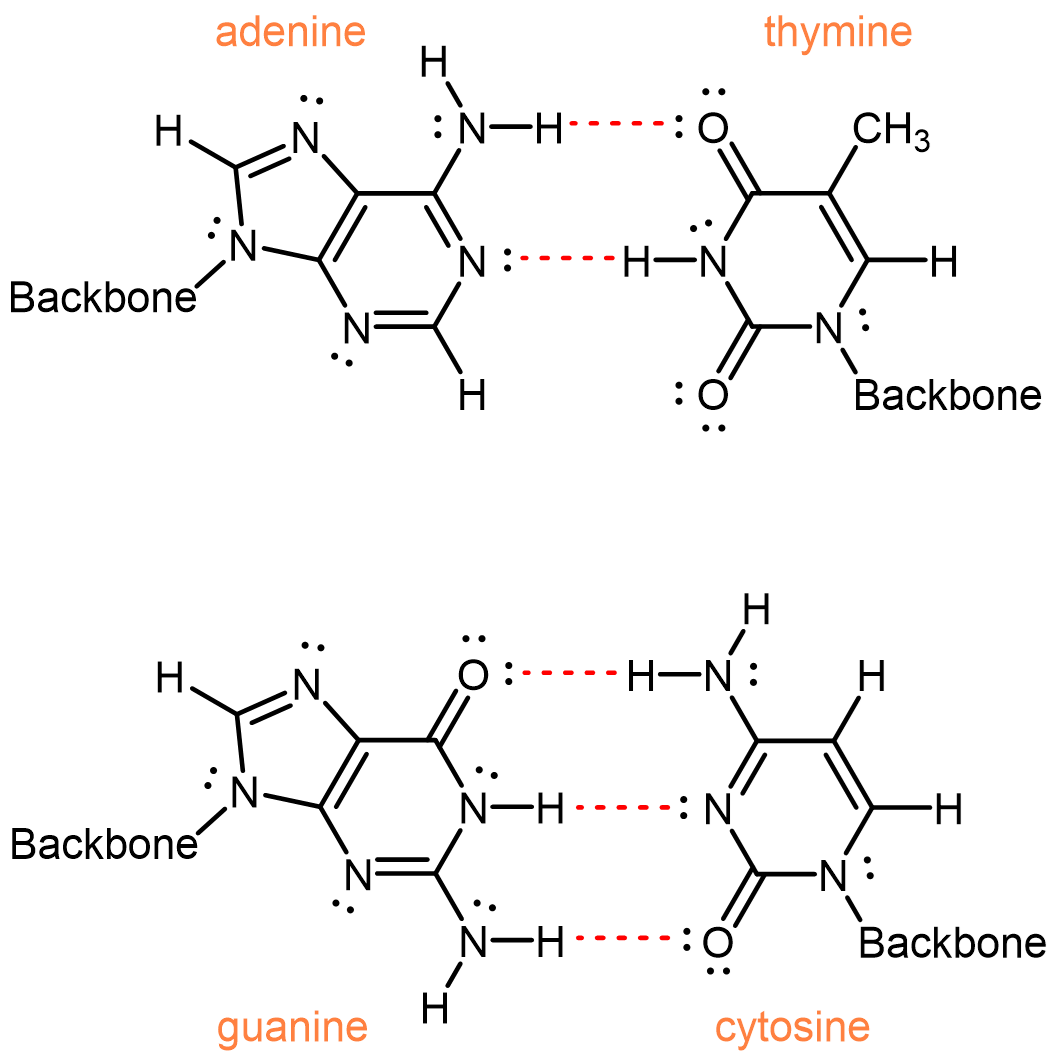
Two single strands of DNA wrap around each other to form two intertwined helixes, the famous double-helix. The two separate strands are held by hydrogen bonding between nucleobases.

One of the most remarkable things about DNA is that the quantity of adenine always equals the quantity of thymine and the quantity of guanine always equals the quantity of cytosine. This imply that the nucleobases occur in pairs: adenine paired with thymine and guanine paired with cytosine. These pairs of nucleobases are called complementary base pairs, and they maximize the number of hydrogen bonds (red dashed bonds) across the shared helical axis:
Glycerolipids
Fats and oils are part of a class of biomolecules called lipids. The molecular structures of lipids give rise to different intermolecular interactions that influence their physical properties. Here, we discuss an important classes of lipids: glycerolipids.
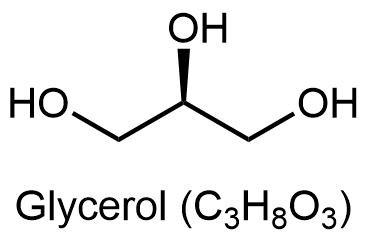
Glycerolipids are composed of glycerol and fatty acids. Glycerol has three carbon atoms, each of which has a hydroxyl (-OH) group bonded to it:
Fatty acids are long, unbranched hydrocarbon chains with a carboxylic acid group at one end.
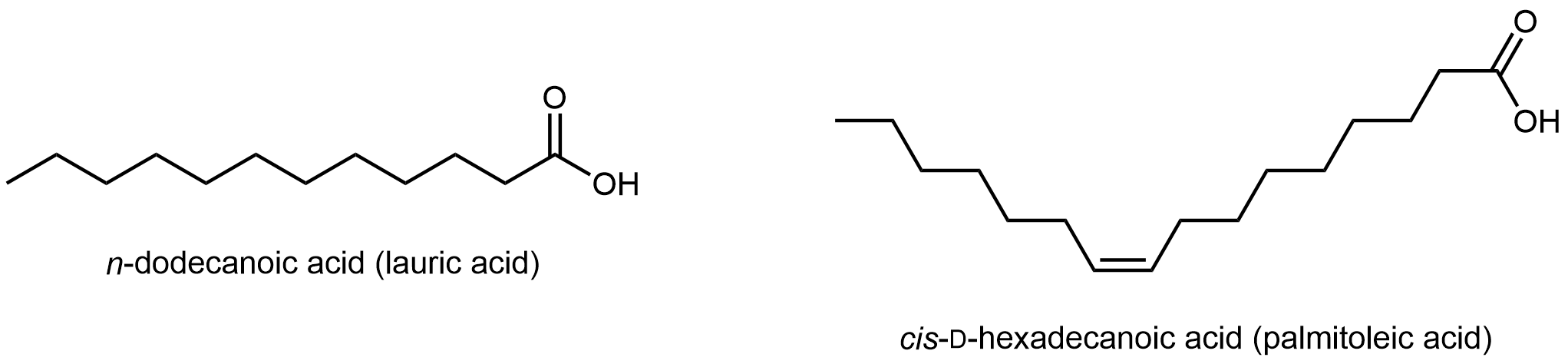
Note that there are only C-C single bonds in lauric acid; similar to hydrocarbons, such a fatty acid is a saturated fatty acid. In contrast, palmitoleic acid has a C=C double bond, i.e. it has one degree of unsaturation. The π bond can undergo addition of H2 and transform into a saturated fatty acid. Lipids made from saturated and unsaturated fatty acids have different properties.
Glycerolipids are formed when three fatty acids are joined to glycerol. Catalyzed by enzymes, each hydroxyl group on glycerol can undergo condensation with the carboxylic acid group of a fatty acid, forming an ester linkage.
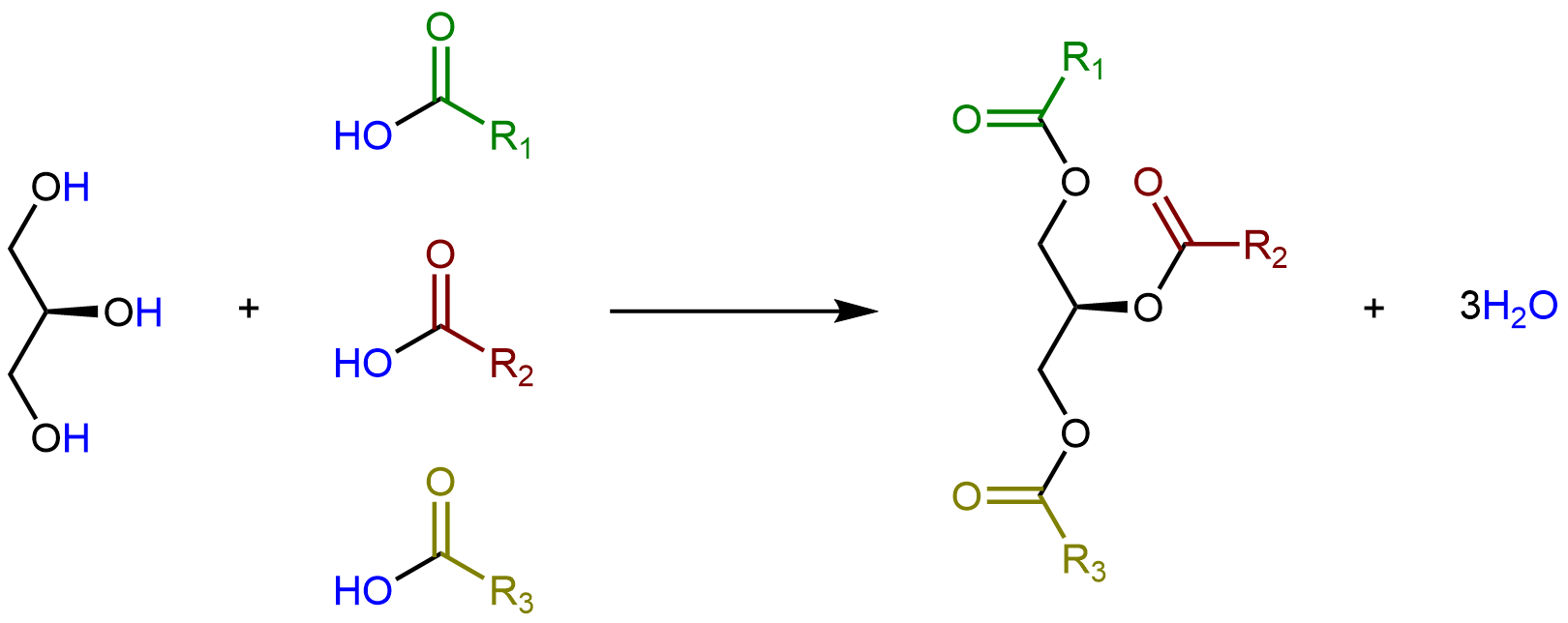
The lengths of the fatty acid chains (e.g. R1, R2, R3) and the number of C=C double bonds within them determine the melting point of a glycerolipid. Longer chains have stronger LDFs than shorter chains, leading to a higher melting point. Saturated chains can pack tightly against each other, giving rise to stronger LDFs between the chains and higher melting points. Unsaturated chains, especially with cis double bonds that force the chain to bend, cannot stack together as well, so the LDFs are weaker.
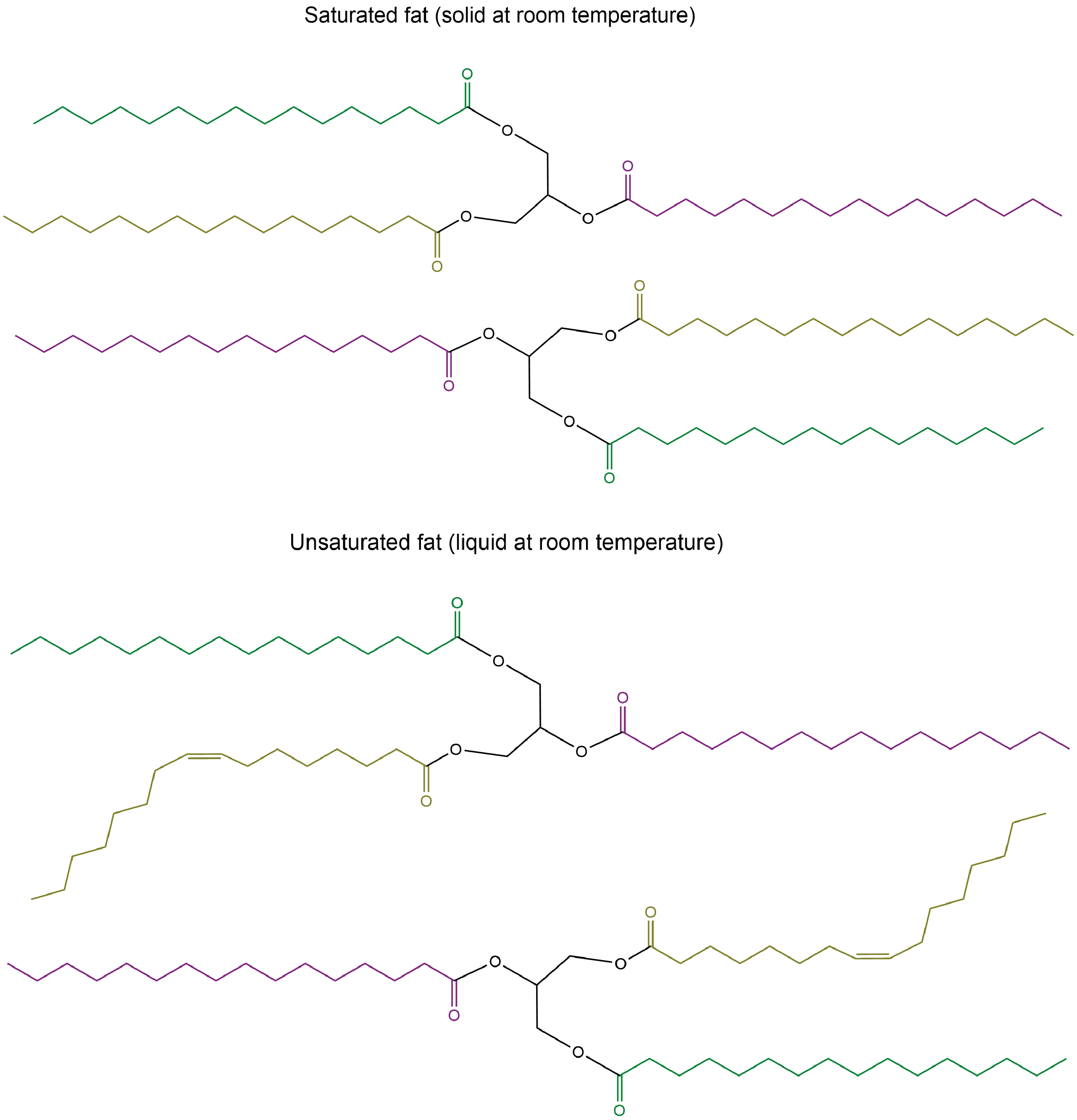
Glycerolipids with a higher melting point that are usually solids at room temperature are called fats or waxes. Glycerolipids with a lower melting point that are usually liquids at room temperature are called oils.
An important role of glycerolipids is in energy storage. The carbon atoms in fatty acids are mostly involved in C-H bonds, and therefore can be oxidized and in the process release energy. In living organisms, this is carried out in a controlled manner via enzymes. But we can also burn fats and waxes (for example, in a candle) to obtain energy in the form of light.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

