D6.4 MOs for Second-row Diatomic Molecules
Let’s consider some slightly more complex examples of molecular orbitals. F2, O2, and N2 are diatomic molecules formed by elements from the second row of the periodic table. These molecules contain many more electrons than H2, and their molecular orbitals are derived from p atomic orbitals as well as s atomic orbitals.
Activity: Shapes and Phases of p Atomic Orbitals
Additional Practice
Now think about what happens when two atoms containing 2p atomic orbitals approach each other. Assume that the internuclear axis is the z axis. This means that the 2pz atomic orbitals are aligned along the internuclear axis while the 2px and 2py atomic orbitals are oriented perpendicular to the internuclear axis.
When the two atoms approach, the bonding and antibonding overlap of the two 2pz atomic orbitals occurs along the internuclear axis (z axis):
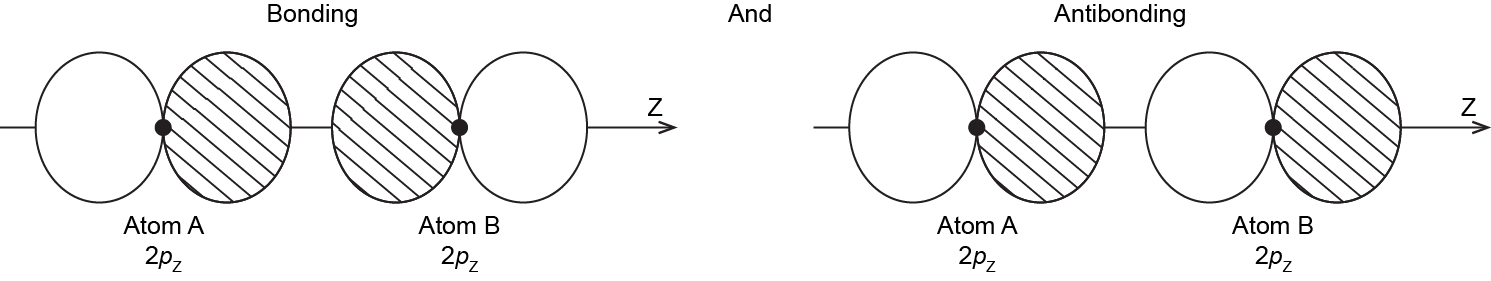
MOs derived from these two combinations are labeled σ2pz and σ*2pz. If you don’t understand why these MOs are have the label σ, review the section on MO diagrams.
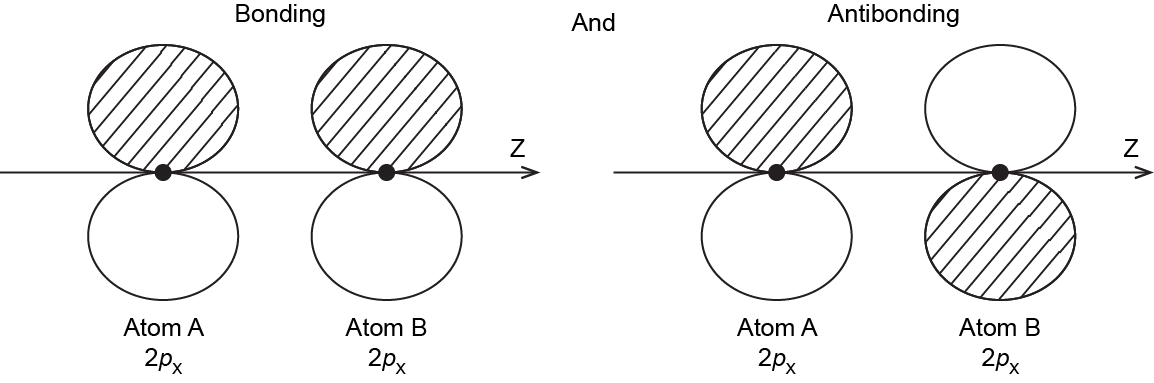
It is also possible to make bonding and antibonding combinations from the two 2px AOs and from the two 2py AOs. Here is a diagram for the two ways the 2px AOs can overlap. Notice that the orbitals overlap side-by-side, not end-on, because the 2px AOs are aligned perpendicular to the internuclear axis (z axis).
Activity: Molecular Orbitals Involving p Atomic Orbitals
Additional Practice
When two 2p AOs overlap side-by-side, the bonding MO formed is not symmetric with respect to rotation around the internuclear axis. Thus, the bond formed is not a σ bond. If you look down the internuclear (bond) axis, the “side view” of the MO looks similar to a 2p atomic orbital; this MO is called a π orbital.
Think about all atomic orbitals that are occupied in a fluorine atom, F: 1s, 2s, 2pz, 2px, and 2py. For each pair of AOs (such as 2s on atom A with 2s on atom B), overlap produces one bonding and one antibonding MO. There is σ and σ* for 1s – 1s, 2s – 2s, and 2pz – 2pz overlaps. There is π and π* for 2px – 2px, and 2py – 2py overlaps. These ideas result in the MO energy-level diagram shown here:
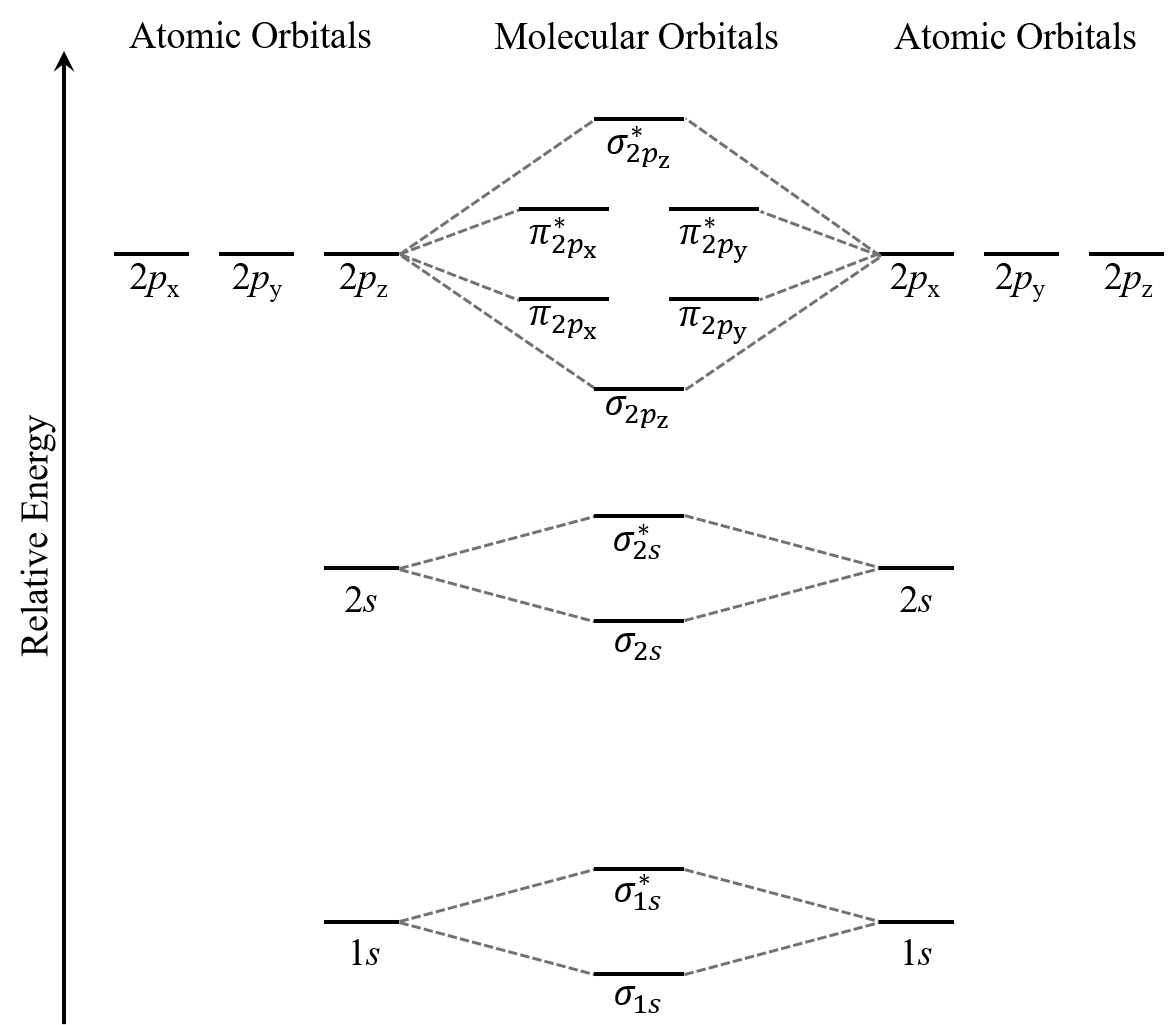
From the ten AOs (five from each F atom), ten MOs are formed in F2. Note that whether a MO is bonding or antibonding is dependent on whether it is lower or higher in energy than the AOs from which it is derived. Hence, even though σ*2s is lower in energy than σ2pz, it is still an antibonding MO.
The y-axis in the above figure only shows qualitative relative energy ordering. If you consider the actual energy values, the core electrons are much much lower in energy than the valence electrons. For example, the energy difference between the 1s and 2s atomic orbitals in F is 62,000 kJ/mol while the difference between 2s and 2p is only 1,800 kJ/mol. The core electrons also occupy orbitals that are much smaller (closer to their nucleus) than valence electrons. Hence, the core electrons from two atoms will not overlap in any significant way even as their valence electrons overlap to form covalent bonds. This is why you will often see the core electrons omitted from MO diagrams. (Such omission will not hinder your analysis of the molecule. For example, results from bond order calculations will be the same with or without core electrons.)
We also see in Figure: MO Energies for F2 that the two π bonding MOs, π2px and π2py, are degenerate (have the same energy). This is because the side-by-side overlap of two 2px AOs is identical to the side-by-side overlap of two 2py AOs. They differ only in that π2px and π2py are perpendicular to each other, because the 2px AO is perpendicular to the 2py AO. Similar reasoning leads to the conclusion that the π*2px and π*2py MOs are also degenerate. Recognizing degenerate MOs is important when applying Hund’s rule to determine molecular electron configurations.
Figure: Pi-Bond MOs (below) shows the formation of the two perpendicular π bonds as two N atoms approach each other. (The two molecular depictions in the figure represent the same N2 molecule: one shows the 2px – 2px orbital overlap and the other shows the 2py – 2py orbital overlap.)
Exercise: Molecular Orbital Electron Configurations
Additional Practice
Based on the electron configuration for the F2 molecule, the π and π* MOs are all filled; there is no net π bond in the molecule. This is reflected in the bond order calculation: F2 has a bond order of 1, corresponding to a single σ bond.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

