D14.3 Carboxylic Acids
A carboxylic acid functional group, -COOH, has a hydroxyl group linked to a carbonyl carbon atom. It differs from an ester in that the non-carbonyl oxygen is bonded to a hydrogen atom rather than an R group. Hence, carboxylic acid groups are found at one end of a molecule.
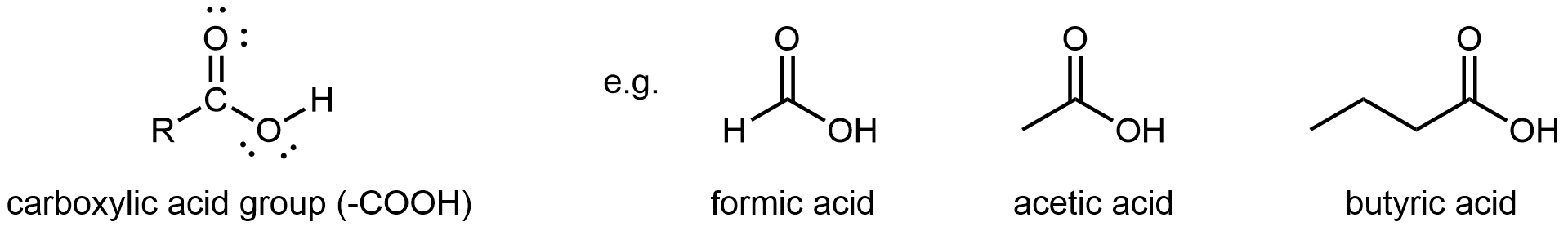
The simplest carboxylic acid is formic acid, known since 1670. Its name comes from the Latin word for ant, formicus. It is partially responsible for the pain and irritation of ants’ and wasps’ stings. Acetic acid is a main component (>4% by volume) of vinegar. Cider vinegar is produced by allowing apple juice to ferment without oxygen present; yeast changes sugar to ethanol, which is then converted to acetic acid via biological oxidation. Butyric acid, a component of rancid butter and Limburger cheese, has a vile odor.
As their name suggests, carboxylic acid groups are acidic. This is because their O-H bond can break relatively easily to yield -COO– and H+, allowing them to donate a proton, H+, to another molecule or ion. This acidity arises from the relative stability of the carboxylate anion, RCOO−.
Activity: Resonance Structures and Acidity of a Carboxylic Acid
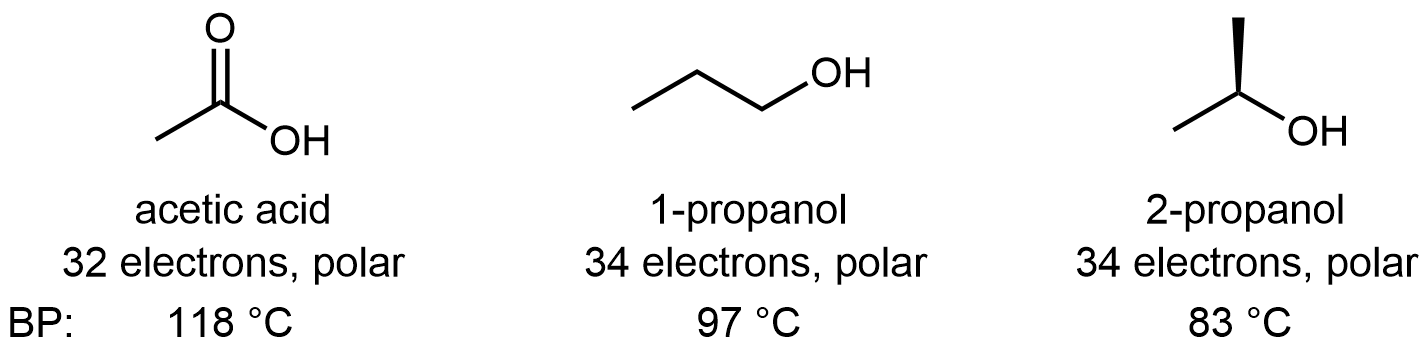
Molecules containing the carboxylic acid functional group are polar and can form hydrogen bonds. Pure acetic acid is called glacial acetic acid because its melting point of 16.6 °C is high enough that it can freeze in a cold laboratory. It is also quite thick and syrupy because the strong hydrogen-bonding attractions between molecules result in high viscosity. The acidity of the carboxylic acid group enhances the O-H···O hydrogen-bond strength, such that hydrogen-bonding between carboxylic acid molecules is usually stronger than between alcohol molecules. For example:
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

