D14.5 Amides
An amide functional group contains a nitrogen atom connected to the carbon atom of a carbonyl group. Like amines, amides can be classified by the number of carbon atoms bonded to the nitrogen:
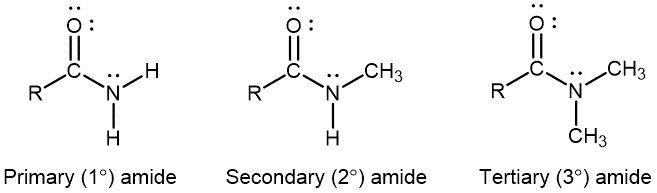
Activity: Amines, Amides, and Resonance Structures
Although the resonance structure with formal charges on O and N does not contribute to the resonance hybrid as much as the resonance structure without formal charges, it is crucial in understanding the chemical and physical properties of amide molecules. For example, the partial double bond character gives rise to a significant energy barrier for rotation about the C-N bond. And because the lone pair on the N atom is part of the π bonding network, the N atom in an amide is about 1010 times less basic than the N atom in an amine.
Additionally, because the lone pair on the N atom is part of the π bonding network, it is not available for hydrogen bonding. However, hydrogen bonds can still form in 1º and 2º amides between the N-H bond(s) and the lone pairs on the O atom.
Exercise: Structures of Amines
Exercise: Recognizing Functional Groups
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

