D38.3 Enzyme Denaturation and Inhibitors
Denaturation is a process in which proteins lose their quaternary, tertiary and secondary structure. Enzymes must be folded into the right 3D shape to function. But noncovalent interactions, which play a crucial part in protein folding, are individually weak, and it does not take much heat, acidity, or other stress to break some of them and denature the enzyme.
Hence, enzyme catalyzed reactions exhibit an unusual temperature dependence. At relatively low temperatures, the reaction rate increases with temperature, as is expected. However, at higher temperatures, the reaction rate drops dramatically due to denaturation of the enzyme, as shown below.

Typically, enzymes will not denature at temperatures encountered by the living organism in which they are found. As a result, enzymes from bacteria living in high-temperature environments such as hot springs are prized by industrial users for their ability to function at higher temperatures.
Exercise: Enzyme Characteristics
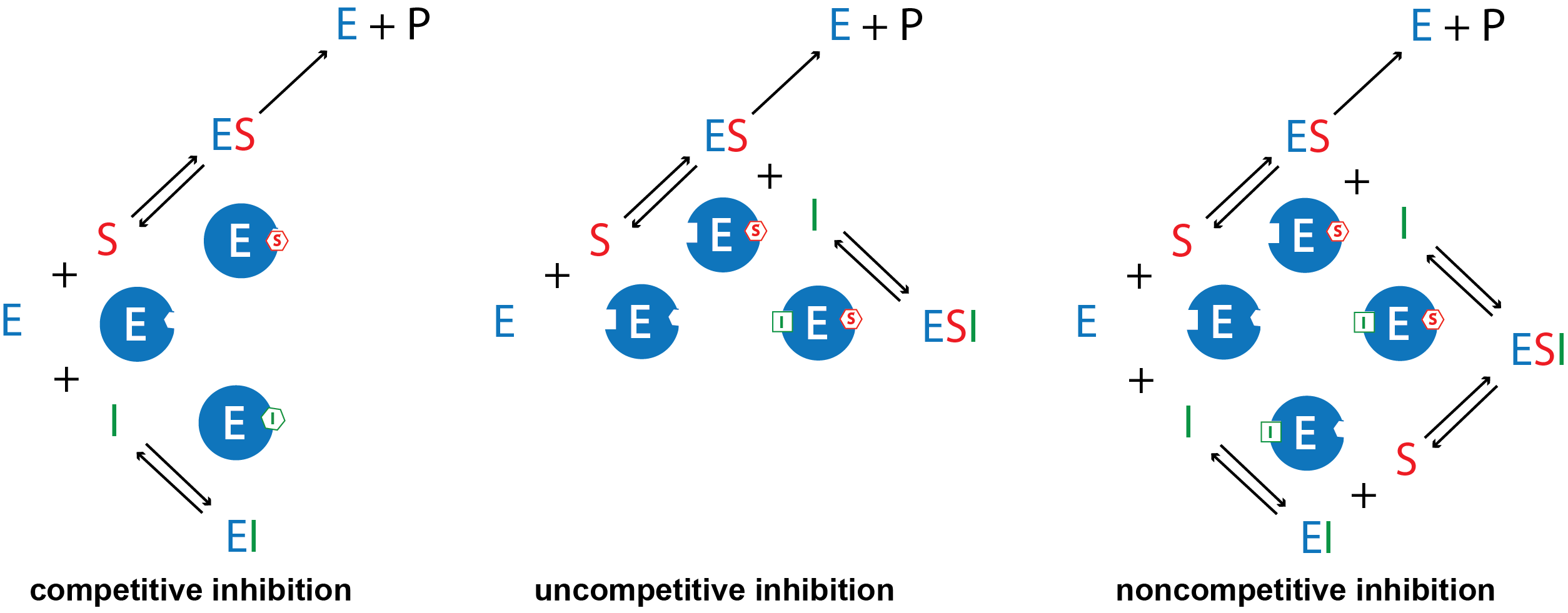
An inhibitor interacts with an enzyme to decreases the enzyme’s catalytic efficiency. An irreversible inhibitor covalently binds to the enzyme’s active site, producing a permanent loss in catalytic efficiency even if the inhibitor’s concentration is later decreased. A reversible inhibitor forms a noncovalent complex with the enzyme, resulting in a temporary decrease in catalytic efficiency. Reducing the concentration of a reversible inhibitor returns the enzyme’s catalytic efficiency to its normal level.
There are several kinds of reversible inhibition. In competitive inhibition the substrate and the inhibitor compete for the same active site on the enzyme. Because the substrate cannot bind to an enzyme–inhibitor (EI) complex, the concentration of enzyme available to form enzyme-substrate (ES) complex is lower and the enzyme-catalyzed reaction is slower. With uncompetitive inhibition the inhibitor binds to the enzyme-substrate (ES) complex but not at the active site, forming an enzyme–substrate–inhibitor (ESI) complex. The formation of an ESI complex decreases catalytic efficiency because it reduces the concentration of ES, which reduces the rate of the rate-limiting step in the mechanism. Finally, in noncompetitive inhibition the inhibitor binds to both the enzyme itself and the enzyme–substrate complex at a site different from the active site. Similar to uncompetitive inhibition, this forms an inactive ESI complex and reduces the concentration of ES.
Activity: Enzyme Inhibition
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

