D26.4 Protein Structure
We have already discussed some basic aspects of protein structure. A protein strand’s primary structure, its sequence of amino acids, dictate the ultimate 3D structure the protein will adopt. While every protein strand has the same backbone (with varying lengths), each protein’s sequence of side chain groups, determined by its sequence of amino acids, is unique.
The secondary structures of proteins, such as α-helix and β-sheet, are stabilized by hydrogen-bonding between backbone amide groups. In an α-helix segment, the side chains project out from the helix, while in a β-sheet segment, the side chains alternate above and below the β-sheet.
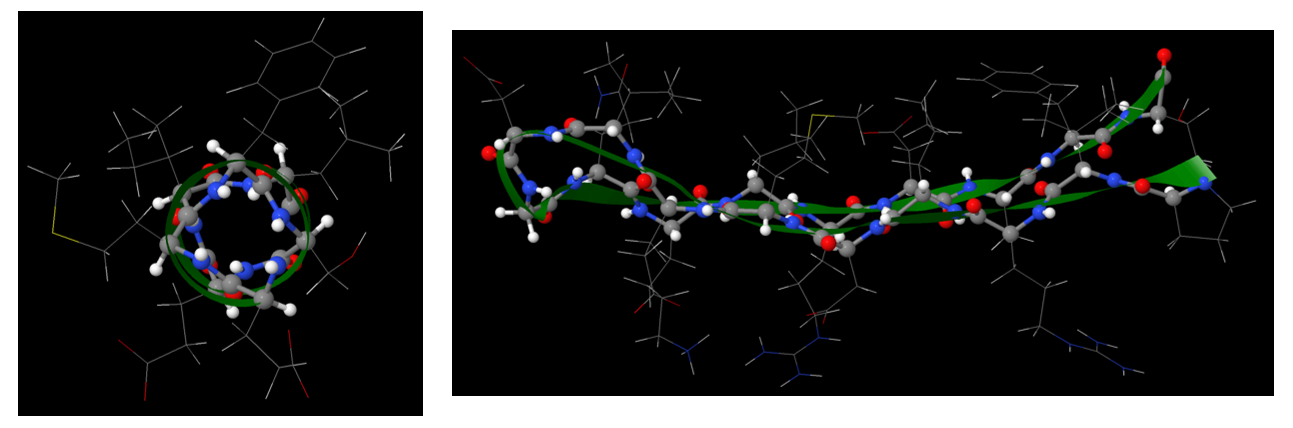
Therefore, when a protein strand adopts its tertiary structure, it is mainly the side chains that are exposed to the surrounding environment. Their hydrophobic or hydrophilic nature directly influences the 3D structure that a protein adopts.
When a protein strand is being synthesized in a cell, it has a random geometry. This state is generally referred to as being “unfolded”. Once the protein strand adopts its proper tertiary structure, it is now “folded”. This folding process:
occurs spontaneously and has an overall negative ΔrG°.
Activity: Thermodynamics of protein folding
Part of the ΔrH° of protein folding comes from the numerous hydrogen bonds formed in the secondary structures. Also important is the noncovalent interactions present between nearby side chains and between side chains and the surrounding solvent. While a protein strand has the possibility to adopt a huge variety of 3D structures, the one specific structure it ultimately adopts is the one with the lowest energy for the environment the protein is in. For example, exposing most hydrophilic side chains to an aqueous environment while protecting most hydrophobic side chains form the same aqueous environment is an energetically favorable configuration.
Two general kinds of proteins are found in cells, water soluble and water insoluble proteins. Water soluble proteins, which include enzymes and transport proteins, are found free in cellular compartments such as the cytoplasm, nucleus, or endoplasmic reticulum.
In the tertiary structure of water-soluble proteins, most hydrophobic side chains are in the interior of the protein and away from water, while most hydrophilic side chains (polar, acidic, or basic) are mostly kept on the exterior and therefore exposed to water. (The backbone amide groups, while polar and capable of forming hydrogen bonds, do not interact much with water because they are already engaged in hydrogen bonding with each other in α helices and β sheets.)
The not water-soluble proteins include proteins that cross lipid bilayers once or more (integral membrane proteins). Integral membrane proteins include membrane channels, pumps, and receptors.
Passage of large molecules or ions through lipid bilayers can be controlled by protein molecules that are embedded in a bilayer and extend outside the bilayer on both sides. In these membrane-spanning proteins, side chains facing the lipid membranes are usually hydrophobic; side chains contacting the aqueous space on either side of the membrane can be hydrophilic.
Many such proteins are anchored by forming a covalent bond to the cell membrane. For example, the amine group (-NH2) on one end of a protein or in a lysine side chain can undergo a condensation reaction with the carboxylic acid group (-COOH) on a fatty acid molecule found in a cell membrane, thus forming an amide bond. Such a covalent bond keeps the protein associated with the membrane.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

