D19.3 Solution and Solubility
Solutions are homogeneous mixtures of two or more substances. Often, one component of a solution is present at a significantly greater concentration, in which case it is called the solvent. The other components of the solution present in relatively lesser concentrations are called solutes.
Intermolecular forces (IMFs) directly affect the solubility of a compound, because when solute molecules are dispersed among solvent molecules, solute-solute IMFs are replaced by solute-solvent IMFs. Let’s use alcohol molecules as an example to consider this in more detail.
The -OH end of an alcohol molecule is hydrophilic (“water-loving”), capable of strong intermolecular interactions with water molecules. In the case of methanol (CH3OH), the dipole-dipole and hydrogen-bond interactions between methanol and water molecules are similar in strength to those between methanol and methanol molecules or between water and water molecules. Therefore, when methanol-methanol (and water-water) IMFs are replaced by methanol-water IMFs, the change in energy (enthalpy change of solution) is minimal; in fact, mixing methanol in water is slightly exothermic.
On the other hand, the hydrocarbon (alkyl) end of an alcohol molecule is essentially nonpolar and is hydrophobic (“water-fearing”), where the intermolecular interactions with water molecules are weak. For example, for hexane, the water-water IMFs and hexane-hexane IMFs are much stronger than hexane-water IMFs. Therefore, forcing hexane to dissolve in water would require significant input of energy (endothermic/positive enthalpy change of solution).
Hence, although the size of the alkyl group has little influence on the reactivity of an alcohol molecule, it has a significant impact on the solubility of alcohols in water. Alcohols with small alkyl groups, e.g. methanol and ethanol, are completely miscible with water, which means they have infinite mutual solubility in water. Alcohols with larger alkyl groups, such as 1-octanol, are immiscible in water, which means that when 1-octanol is added to water two layers form. One layer is nearly pure water and the other layer nearly pure 1-octanol, because the solubility of each substance in the other is very low.
This is similarly true for the other polar and hydrogen-bonding functional groups. For example, acetic acid is miscible with water, while octanoic acid is immiscible with water.
The situation is slightly different when an ionic compound is added to water. For example, when solid potassium chloride, KCl, is added to water, the compound dissolves and dissociates to yield K+ and Cl– uniformly distributed throughout the mixture:
An ionic compound that does not dissolve in water will remain in its solid state when added to water.
Similar to the alcohol example, it is the IMFs, as well as the lattice energy of the ionic compound, that directly affect the solubility. Dissolving an ionic compound involves breaking apart the ionic lattice (an endothermic process), together with forming new water-ion interactions (an exothermic process).
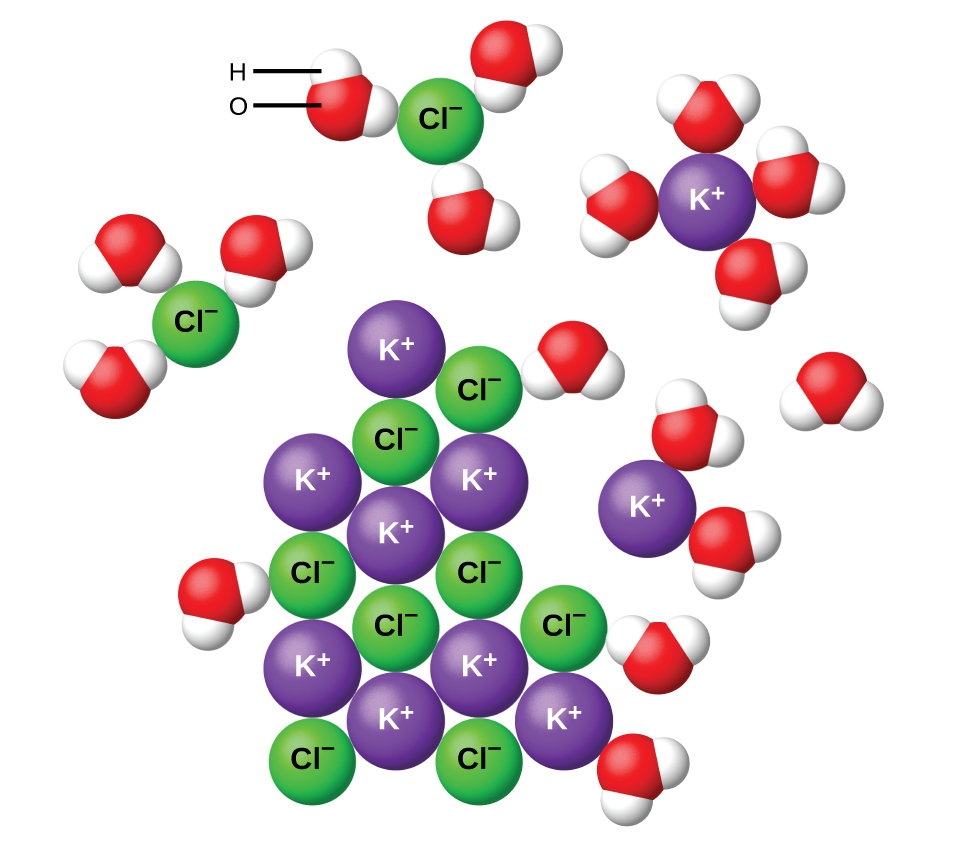
The lattice energy and the water-ion interaction strength (solvation energy) are often comparable in magnitude. Therefore, dissolving an ionic compound in water is usually slightly endothermic or slightly exothermic overall. Ionic compounds that dissolve exothermically are generally quite soluble. On the other hand, ionic compounds that dissolve endothermically are not always insoluble (see video below). This indicates that a factor other than enthalpy is also at play here. This other property is called entropy.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

