D14.4 Amines
An amine functional group is a derivative of ammonia that contains one or more carbon-nitrogen bonds.
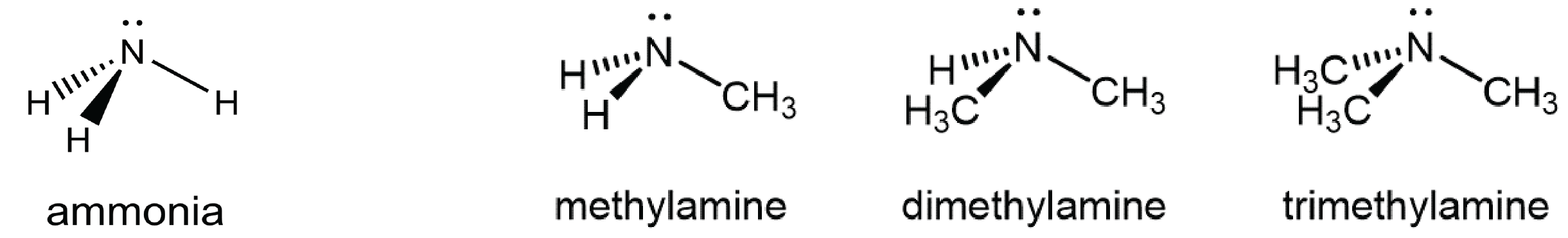
You can classify amine molecules by the number of C-N bonds they contain. In the above example, methylamine is a primary (1º) amine, dimethylamine is a secondary (2º) amine, and trimethylamine is a tertiary (3º) amine. In this figure methyl groups are shown but any R group, such as ethyl, could replace any of the methyl groups.
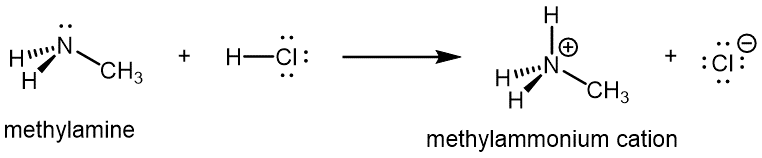
Like ammonia, amines are basic due to the lone pair on the nitrogen atom, and can undergo acid-base reactions to form protonated amine cations analogous to the ammonium ion NH4+:
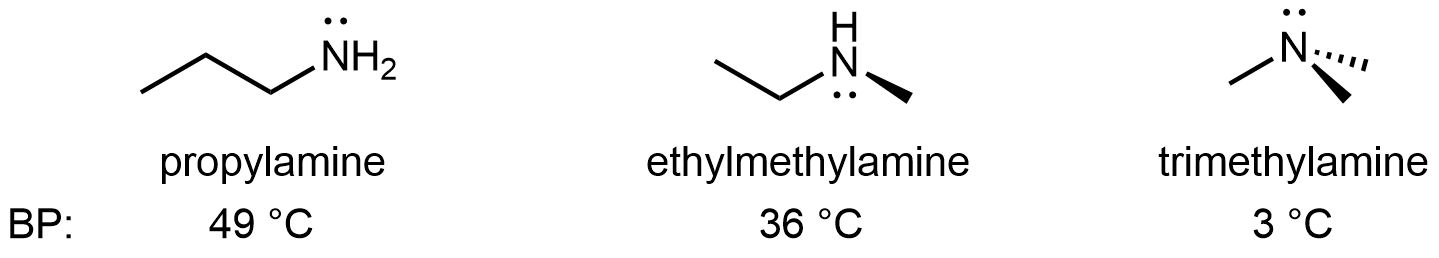
For amines, 1º and 2º amines are capable of hydrogen bonding due to the presence of a N-H bond and the lone pair on the N atom, but 3º amines cannot form hydrogen bonds. For example, for the three amine isomers shown below, the boiling point of the 3º amine is significantly lower than the 1º and 2º isomers.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

