D24.3 Acid Constant and Base Constant
The relative strengths of Brønsted-Lowry acids and bases can be evaluated by comparing the equilibrium constants for their ionization reactions. For the reaction of a generic acid, HA, in water:
we write the acid ionization equilibrium constant (Ka) expression as:
(Although water is a reactant in the reaction, it is also the solvent with its phase indicated as “ℓ”, so we do not include [H2O] in the expression.)
A stronger acid, which ionizes to a greater extent, would have a larger Ka compared to a weaker acid. In other words, compared to an acid with a smaller Ka, an acid with a larger Ka would have a higher concentration of H3O+ and A− relative to the concentration of the nonionized acid, HA, at equilibrium.
For example, these data on acid ionization constants:
| CH3COOH(aq) + H2O(ℓ) | ⇌ | CH3COO–(aq) + H3O+(aq) | Ka = 1.8 × 10-5 |
| HNO2(aq) + H2O(ℓ) | ⇌ | NO2–(aq) + H3O+(aq) | Ka = 7.4 × 10-4 |
| HSO4–(aq) + H2O(ℓ) | ⇌ | SO42-(aq) + H3O+(aq) | Ka = 1.1 × 10-2 |
indicate that the order of acid strength is: acetic acid (CH3COOH) is a weaker acid than nitrous acid (HNO2) which is a weaker acid than hydrogen sulfate ion (HSO4–).
We can consider the strength of a base (B) similarly by considering the extend that it will form hydroxide ions in an aqueous solution:
where the base ionization equilibrium constant (Kb) expression is:
A stronger base ionizes to a greater extent than a weaker base. Therefore, a stronger base has a larger Kb than a weaker base.
Notice that Ka and Kb provide a quantitative measure of acid and base strengths—significantly more accurate than qualitative descriptions of “strong acid” or “weak acid”.
Consider the ionization reactions for a conjugate acid base pair, HA and A−:
A–(aq) + H2O(ℓ) ⇌ HA(aq) + OH–(aq)
Adding these two chemical equations yields the equation for the autoionization of water:
Therefore:
For example, at 25 °C, Ka of acetic acid (CH3COOH) is 1.8 × 10−5 M, and Kb of its conjugate base, acetate anion (CH3COO–), is 5.6 × 10−10 M. The product of these two equilibrium constants is indeed equal to Kw:
This relationship tells us that stronger acids form weaker conjugate bases, and weaker acids form stronger conjugate bases.
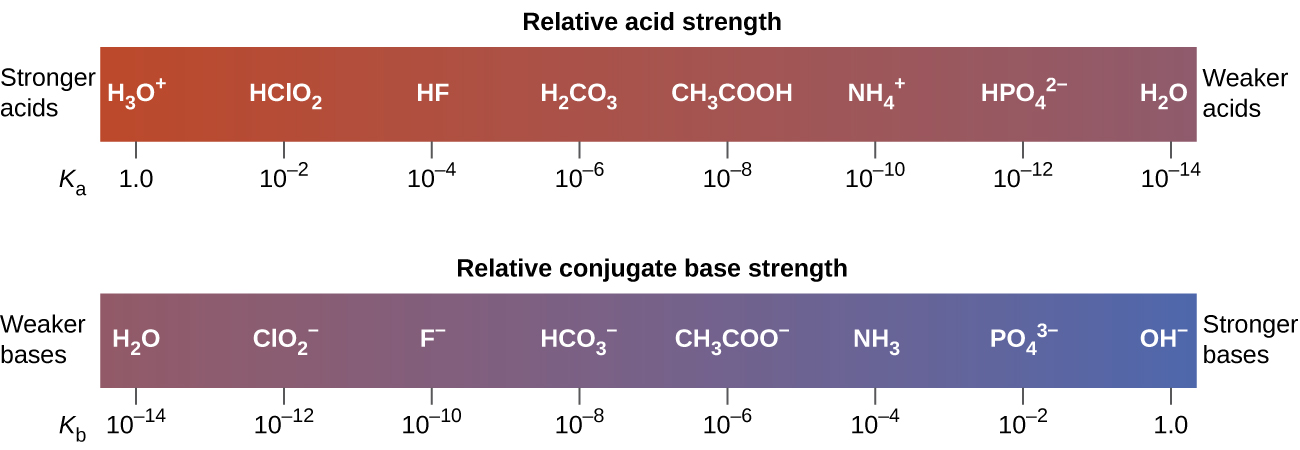
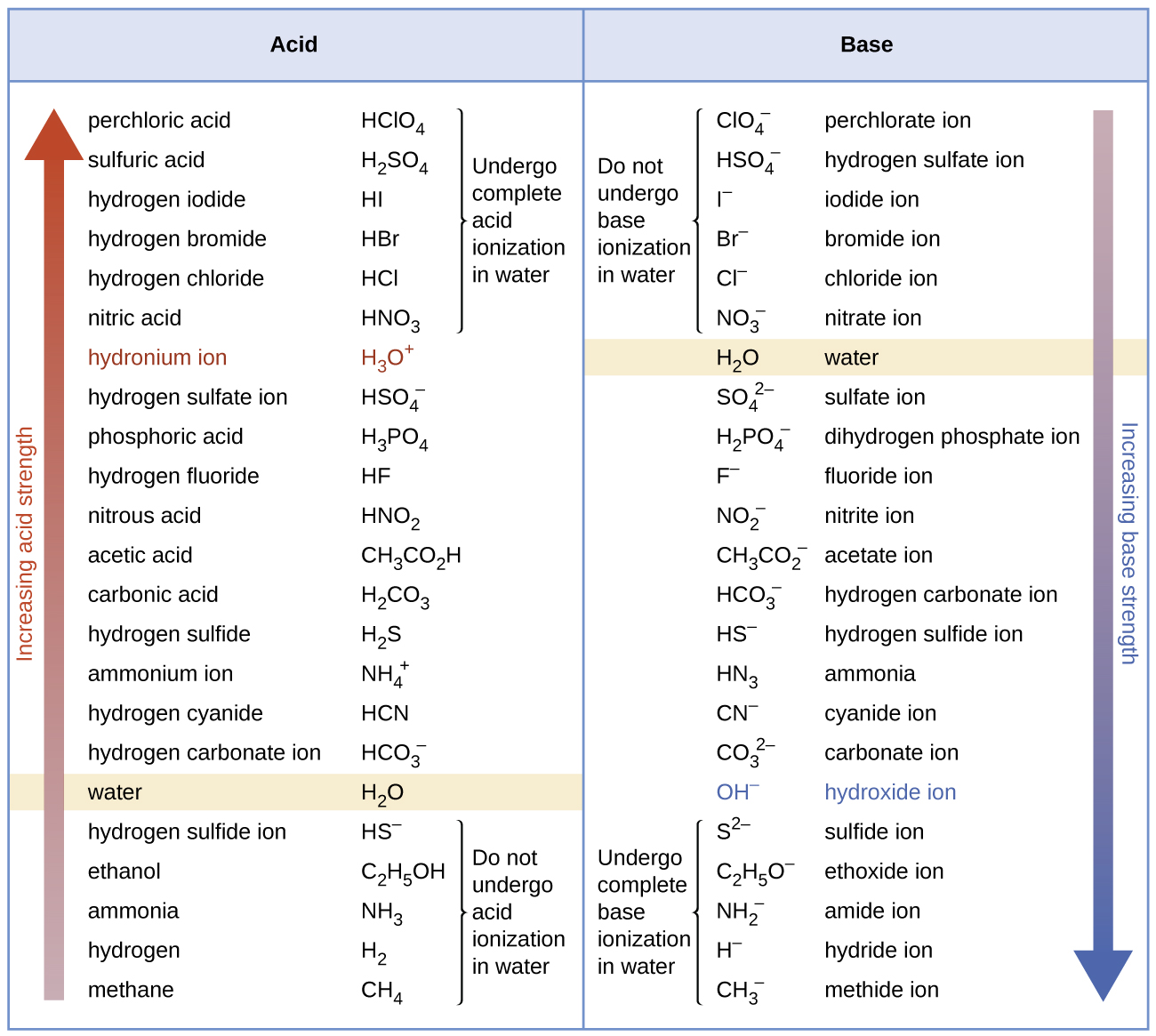
Although “strong” and “weak” are relative terms, we generally refer to acids stronger than H3O+ as strong acids, and bases stronger than OH– as strong bases. Because strong acids and strong bases are completely ionized in aqueous solutions, the concentration of nonionized acid or base is essentially zero. For example, in a 0.10-M solution of HCl, [HCl]e = 0, [H3O+]e = 0.10 M, and [Cl−]e = 0.10 M.
A consequence of this complete ionization is that in aqueous solution there is no way to tell whether one strong acid is stronger than another: HCl, HBr, and HI all are completely ionized. This is known as the leveling effect of water. However, when dissolved in other solvents, these acids do not ionize completely. The extent of ionization increases in the order HCl < HBr < HI, and so HI is the strongest of these acids. Water exerts a similar leveling effect on strong bases.
Many acids and bases are considered “weak”. A solution of a weak acid in water is an equilibrium mixture of the nonionized acid, hydronium ion, and the conjugate base of the acid.
Exercise: Brønsted-Lowry Acids and Bases
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

