D20.6 Chemical Equilibrium
A chemical reaction is usually written with a single arrow, which suggests it proceeds in one direction, the direction of the arrow. But all chemical reactions are reversible, and both the forward and reverse reaction occur simultaneously. When reactions involve gases or solutions, where concentrations change as the reaction proceeds, the reaction eventually reaches a dynamic chemical equilibrium.
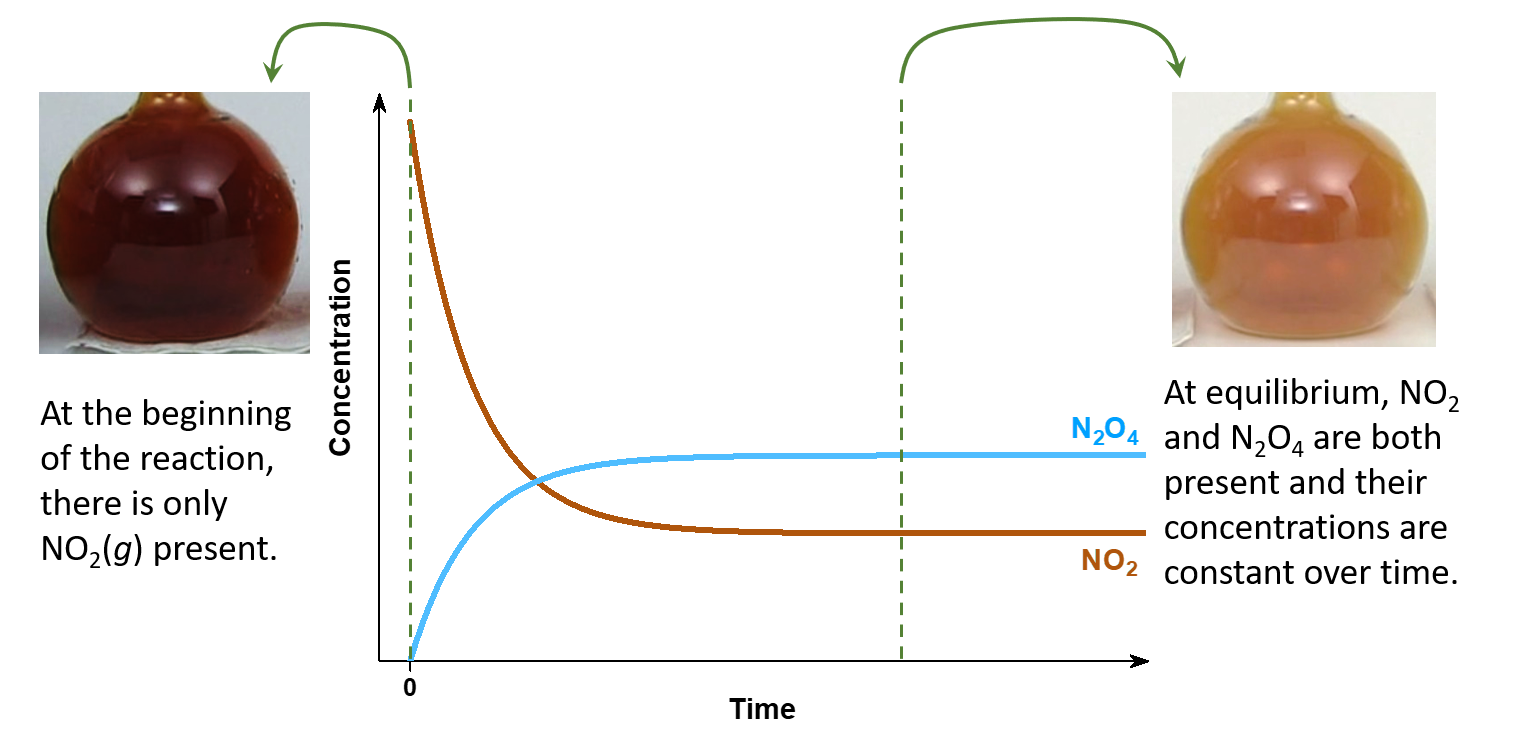
In a chemical equilibrium, the forward and reverse reactions occur at the same rates, and the concentrations of products and reactants remain constant over time. This implies that, if a reaction occurs in a closed system so that the products cannot escape, the reaction often does not yield 100% products. Instead, some reactants remain after the concentrations stop changing. At this point, when there is no further change in concentrations of reactants and products, we say the reaction is at equilibrium.
For example, when we place a sample of nitrogen dioxide (NO2, a red-brown gas) in a glass vessel, the color becomes lighter as NO2 is converted to dinitrogen tetraoxide (N2O4, a colorless gas) by the reaction:
We use the ⇌ arrow when writing an equation for a reversible reaction. (Such a reaction may or may not be at equilibrium.) When we wish to speak about one particular aspect of a reversible reaction, we can use a single arrow.
Activity: Reaction Energies
For the reaction:
At the beginning, there is pure NO2, and only the forward reaction:
is occurring. However, as soon as the forward reaction produces some N2O4, the reverse reaction:
begins to occur, and N2O4 starts to react and form NO2.
At equilibrium, [N2O4] and [NO2] no longer change over time because the rate of the forward reaction is the same as the rate of the reverse reaction, but chemical equilibrium is a dynamic process: the numbers of reactant and product molecules remain constant, but the forward and reverse reactions do not stop.
An equilibrium can be established for a physical change as well as for a chemical reaction. For example:
The figure below shows a sample of liquid Br2 at equilibrium with Br2 vapor in a closed container. When we pour liquid Br2 into an empty bottle in which there is no bromine vapor, some liquid evaporates: the amount of liquid decreases and the amount of vapor increases. If we seal the container so no vapor escapes, the amount of liquid and vapor will eventually stop changing; at that point an equilibrium between the liquid and the vapor has been established. If the container were not sealed, the bromine vapor would escape and no equilibrium would be reached.

Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

