D41.3 Concentration Cells
A concentration cell is a special type of voltaic cell where the electrodes are the same material but the half-cells have different concentrations of soluble species. Because one or both half-cells are not under standard-state conditions, the half-cell potentials are unequal, and there is a potential difference between the half-cells. That potential difference can be calculated using the Nernst equation.
For example, consider this concentration cell at 25 °C:
The standard cell potential is 0 V because the anode and cathode involve the same reaction. However, if equal volumes of the two half-cell solutions were mixed, the concentration of Zn2+ would change to the average of the initial concentrations, namely, to 0.30 M. The cell can do work because the concentrations of Zn2+ change.
| Oxidation: | Zn(s) | ⟶ | Zn2+(aq, 0.10 M) + 2e‾ | E°anode = -0.763 V |
| Reduction : | Zn2+(aq, 0.50 M) + 2e‾ | ⟶ | Zn(s) | E°cathode = -0.763 V |
| Overall: | Zn2+(aq, 0.50 M) | ⟶ | Zn2+(aq, 0.10 M) | E°cell = 0 V |
The Nernst equation verifies that the process is spontaneous at the given conditions, because it shows that Ecell > 0 V:
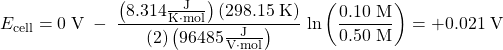
In a concentration cell, the standard cell potential (E°cell) is always zero. In order to have a spontaneous forward reaction, and hence have a positive cell potential (Ecell), the reaction quotient Q must be less than 1 (when Q < 1, ln(Q) < 0). As the reaction proceeds, the concentrations change, Q approaches 1 and Ecell approaches 0 V.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

