D35.3 Elementary Reactions
A balanced equation for a chemical reaction indicates which substances react, which substances are produced, and how the amounts of reactants and products are related. But it does not necessarily show what is happening on the atomic scale as the reaction takes place. Although it is not obvious on the laboratory scale, most chemical reactions occur as a series of atomic-scale steps; that is, as a sequence of simpler reactions, each of which often involves collisions between molecules. The step-by-step sequence of simple reactions by which an overall reaction occurs is called a reaction mechanism.
For example, ozone in the stratosphere protects Earth’s surface from harmful ultraviolet radiation. Ultraviolet photons cause ozone molecules to decompose to oxygen molecules. The overall reaction equation is:
However, at the atomic scale this reaction does not involve collision and reaction between two O3 molecules. Rather, there are two steps that occur one after the other:
| step 1: | O3(g) | O2(g) + O(g) | |
| step 2: | O(g) + O3(g) | ⟶ | 2 O2(g) |
| overall: | 2 O3(g) | ⟶ | 3 O2(g) |
In step 1, upon absorption of an UV photon, a bond breaks in an O3 molecule, producing an O2 molecule and an O atom:
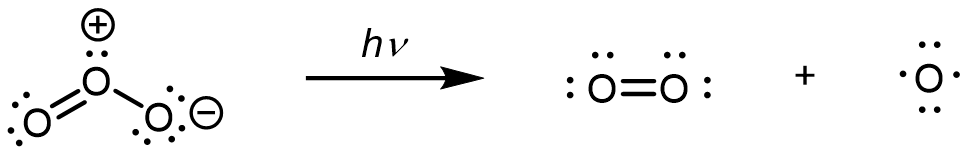
In step 2, the O atom formed in step 1 reacts with a second O3 molecule, producing two O2 molecules:
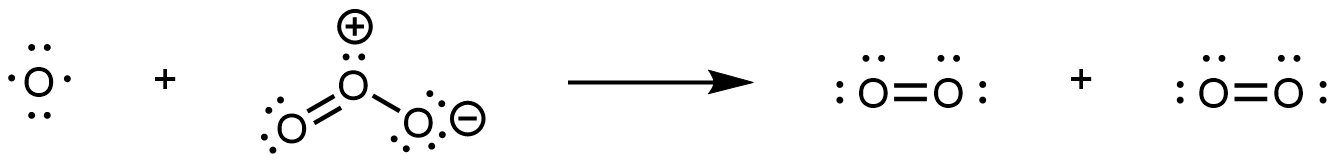
The overall reaction is the sum of these two steps. Write reactants from all steps to the left of a reaction arrow; write products from all steps to the right of the arrow; then cancel formulas that appear on both sides of the arrow. This gives:
which is the overall reaction equation given above: 2 O3(g) ⟶ 3 O2(g). An atom or molecule that is a product in an earlier step and reacts away in a later step of a reaction mechanism is called a reaction intermediate.
Each step in a reaction mechanism is called an elementary reaction, which is a chemical reaction that has only a single transition state. An elementary reaction shows which atomic-scale particles collide, break apart, or rearrange their structures to form reaction products and/or intermediates.
The reaction equation for an elementary reaction specifies exactly which atoms or molecules are involved in that reaction. For example, step 2 in the ozone decomposition reaction mechanism above states that one O atom reacts with one O3 molecule and two O2 molecules are formed. That is, for this elementary reaction to occur, one O atom must collide with one O3 molecule.
In contrast, the overall reaction equation does not necessarily specify which atoms or molecules collide and react. For example, even though the overall ozone reaction is 2 O3(g) ⟶ 3 O2(g), there is no need for two O3 molecules to collide in order for products to form.
Because each elementary reaction has a single transition state, in an overall reaction that consists of several sequential elementary reaction steps, there is a series of transition states, one for each step in the mechanism. For example, the figure below shows the overall reaction energy diagram for the ozone decomposition reaction.
Note that although one of the O3 molecules does not react until step 2, you still need to include it as a reactant in the reaction energy diagram. In other words, in a reaction energy diagram, all the atoms and molecules that are involved in the reaction are accounted for from the very beginning to the very end. Therefore, step 1 becomes 2 O3(g) ⟶ O2(g) + O(g) + O3(g), and step 2 becomes O2(g) + O(g) + O3(g) ⟶ 3 O2(g). This is because there is a significant quantity of energy associated with each atom or molecule; to omit one, or to suddenly add one in the middle of the reaction energy diagram, would significantly change the energy (y-value) associated with that point along the reaction progress.
Exercise: Reaction Mechanism
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

