D13.6 Esters
An ester functional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. A general ester structure has an R group bonded to the carbonyl carbon atom and another R group bonded to the second oxygen. It is a functional group that is found in the middle of a molecule.
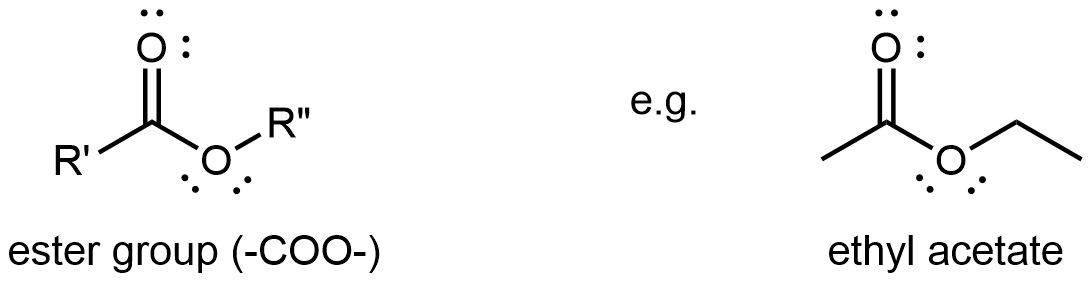
The ester functional group’s carbon atom is sp2 hybridized with a trigonal planar local geometry. Its carbonyl oxygen is sp hybridized, and one of its unhybridized 2p atomic orbitals forms the π bond with the carbon’s unhybridized 2p atomic orbital. This oxygen also has two lone pairs: one occupies a sp hybrid orbital; the other occupies a 2p atomic orbital that is perpendicular to the π bond. The second oxygen (non-carbonyl oxygen) is sp2 hybridized and has a bent local geometry. It also has two lone pairs, one in a sp2 hybrid orbital, the other in the unhybridized 2p atomic orbital.
As shown in the preceding figure, the 2p lone pair on the non-carbonyl O is aligned parallel to the 2p orbitals that form the π bond. This leads to some delocalization of the lone pair electron densities, which can be expressed by resonance structures:
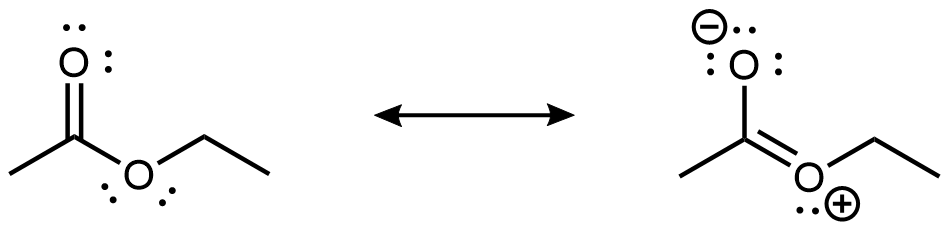
While the resonance structure on the right makes only a fairly minor contribution to the description of the ester molecule, that structure is helpful in understanding the ester’s chemical and physical properties. For example, the -COO- ester group is planar, and the non-carbonyl C-O bond is not as freely rotatable as a typical single bond. Moreover, an ester’s reactivity is quite different from that of a ketone or an ether, and hence an ester is a distinct functional group.
Activity: Ester Hybridization and Local Bond Geometry
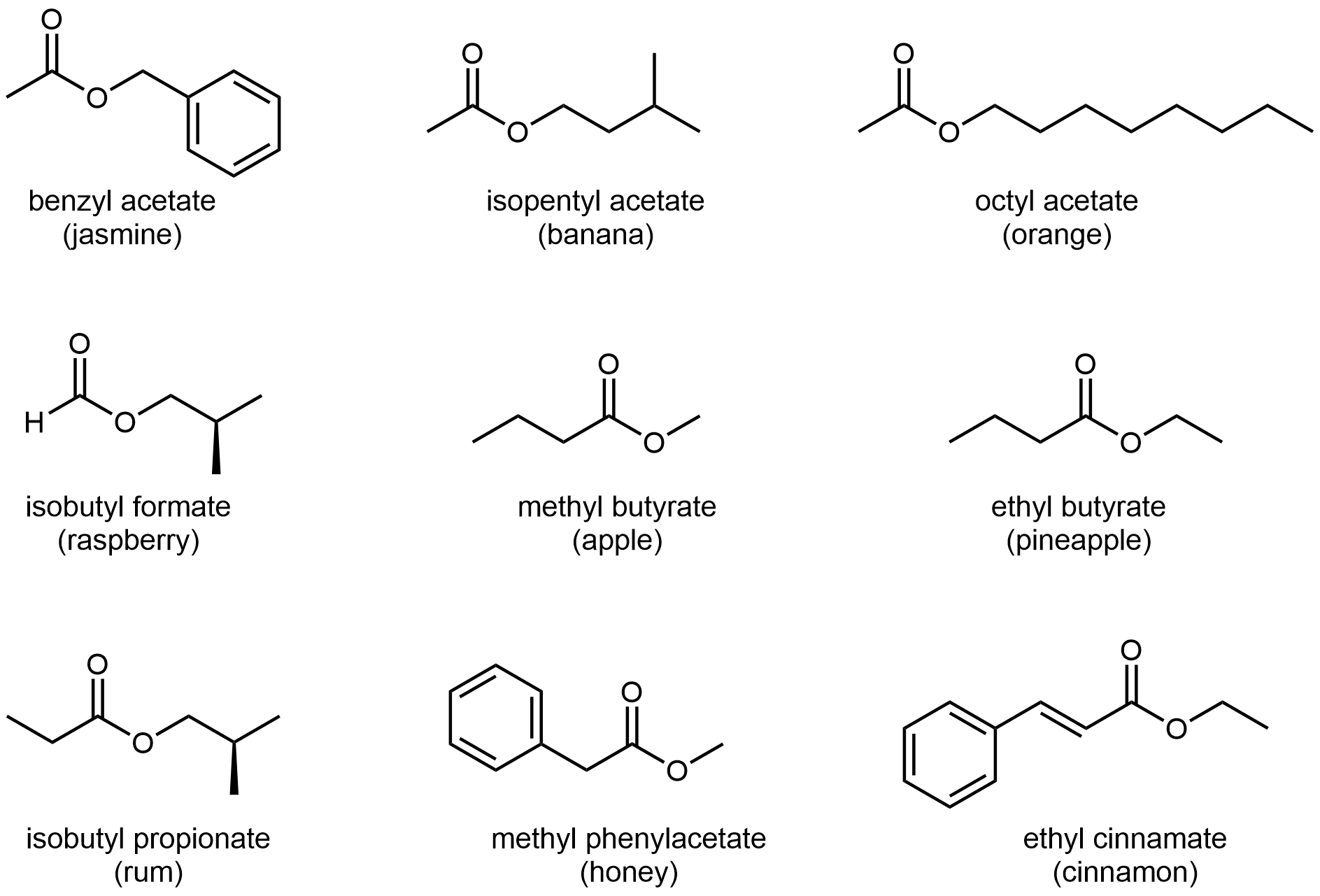
The odors of ripe bananas and many other fruits are due to the presence of esters.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

