D42.5 Fuel Cells
Suppose a voltaic cell is constructed such that the substance that is oxidized at the anode and the substance that is reduced at the cathode (the reactants in the overall redox reaction) are both supplied continuously. Such a battery would never run down because reactant concentrations or partial pressures would never decrease. Such a device is a fuel cell, which produces electricity as long as fuel is available. Hydrogen fuel cells have been used to supply power for satellites, space capsules, automobiles, boats, and submarines.
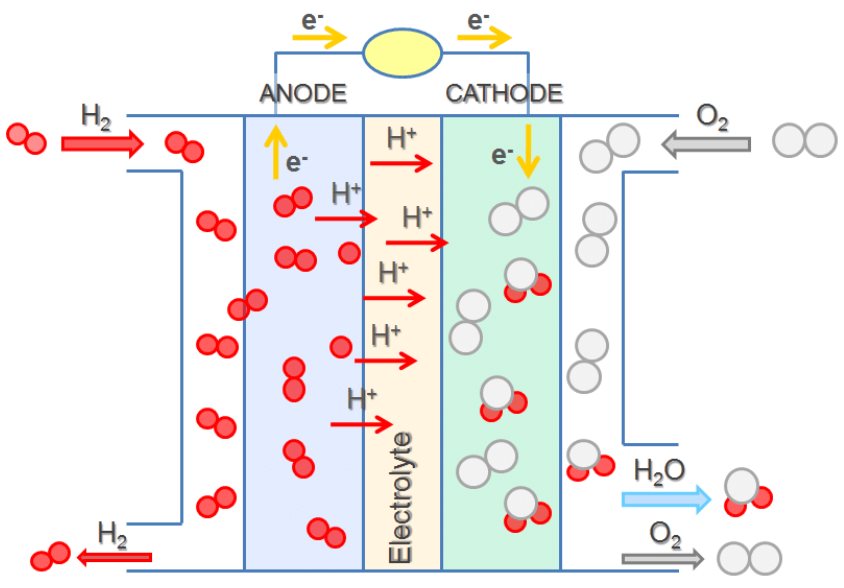
In a hydrogen-oxygen proton-exchange fuel cell, the cell potential is about 1 V, and the reactions involved are:
| Oxidation (anode): | 2 × [H2(g) | ⟶ | 2 H+(aq) + 2 e–] |
| Reduction (cathode): | O2(g) + 4 H+(aq) + 4 e– | ⟶ | 2 H2O(ℓ) |
| overall: | O2(g) + 2 H2(g) | ⟶ | 2 H2O(ℓ) |
The efficiency of fuel cells is typically about 40-60%, which is higher than the typical internal combustion engine (25-35%). Moreover, in the case of the hydrogen fuel cell, nearly pure water is produced as exhaust. Currently, fuel cells are comparably more expensive and contain features that may cause a higher failure rate.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

