D19.1 Hess’s Law
The enthalpy change for a particular reaction is the same regardless of which or how many steps the reaction is carried out in. This conveniently allows us to calculate the enthalpy change for a chemical reaction from other experimentally determined enthalpy changes. This type of calculation usually involves Hess’s law: if a process can be written as the sum of several stepwise processes, the enthalpy change of the total process equals the sum of the enthalpy changes of the various steps.
For example, we can think of the following reaction occurring in a single step:
or in a two-step process:
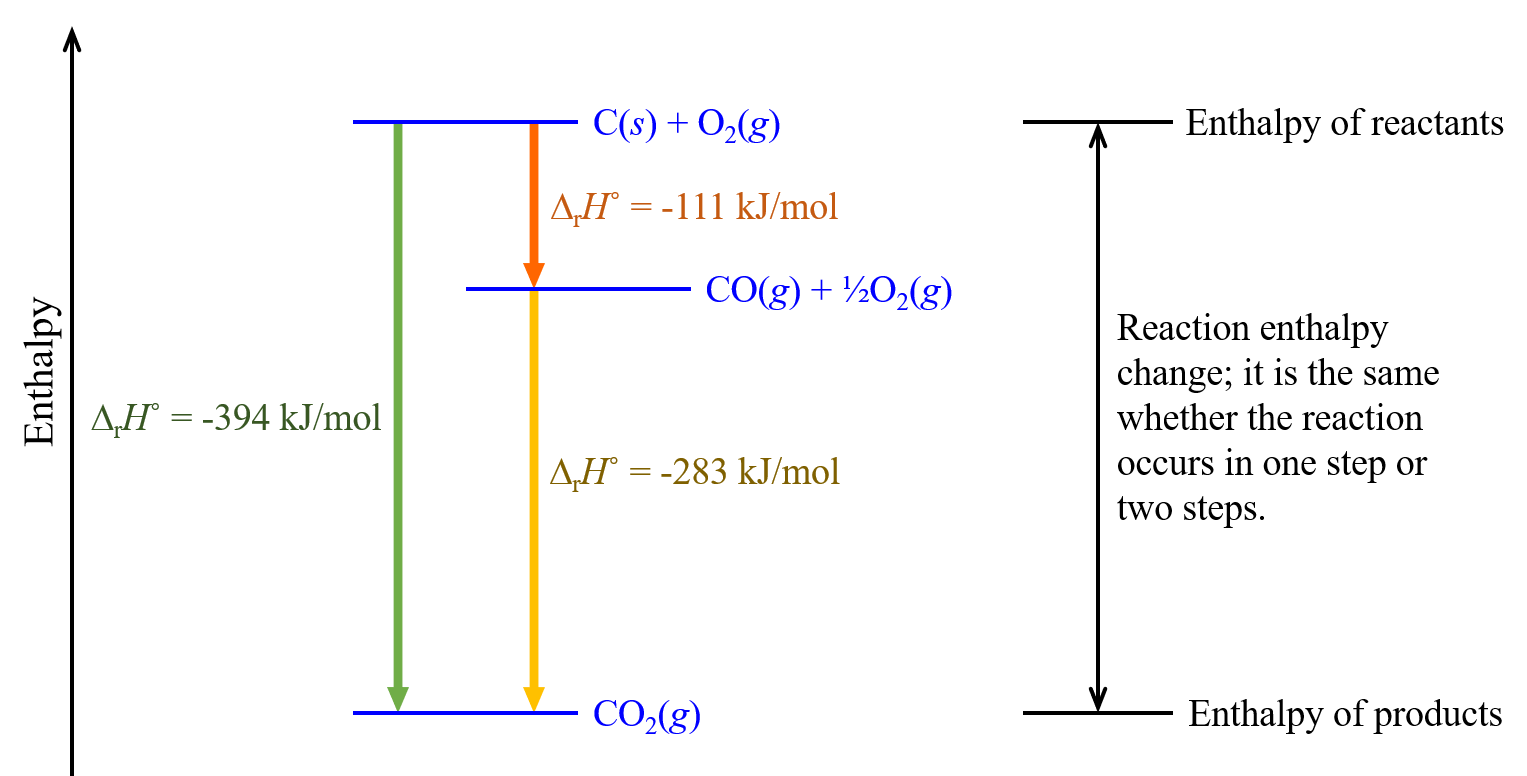
| step 1: | C(s) + ½O2(g) | ⟶ | CO(g) | ΔrH° = -111 kJ/mol |
| step 2: | CO(g) + ½O2(g) | ⟶ | CO2(g) | ΔrH° = -283 kJ/mol |
According to Hess’s law, the ΔrH° of the one-step reaction equals the sum of the ΔrH° of the two steps in the two-step reaction:
| step 1: | C(s) + ½O2(g) | ⟶ | CO(g) | ΔrH° = -111 kJ/mol |
| step 2: | CO(g) + ½O2(g) | ⟶ | CO2(g) | ΔrH° = -283 kJ/mol |
| Sum: | C(s) + O2(g) | ⟶ | CO2(g) | ΔrH° = -394 kJ/mol |
To think about this pictorially, consider the following figure.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

