D23.2 Le Châtelier’s Principle: Change in Concentration
For a chemical system at equilibrium at constant temperature, if the concentration of a reactant or a product is changed, therefore changing Q, the system is no longer at equilibrium because Q ≠ K. The concentrations of all reaction species will then undergo additional changes until the system reaches a new equilibrium with a different set of equilibrium concentrations. We say that the equilibrium shifts in a direction (forward or reverse) that partially counteracts the change.
For example, consider the chemical reaction:
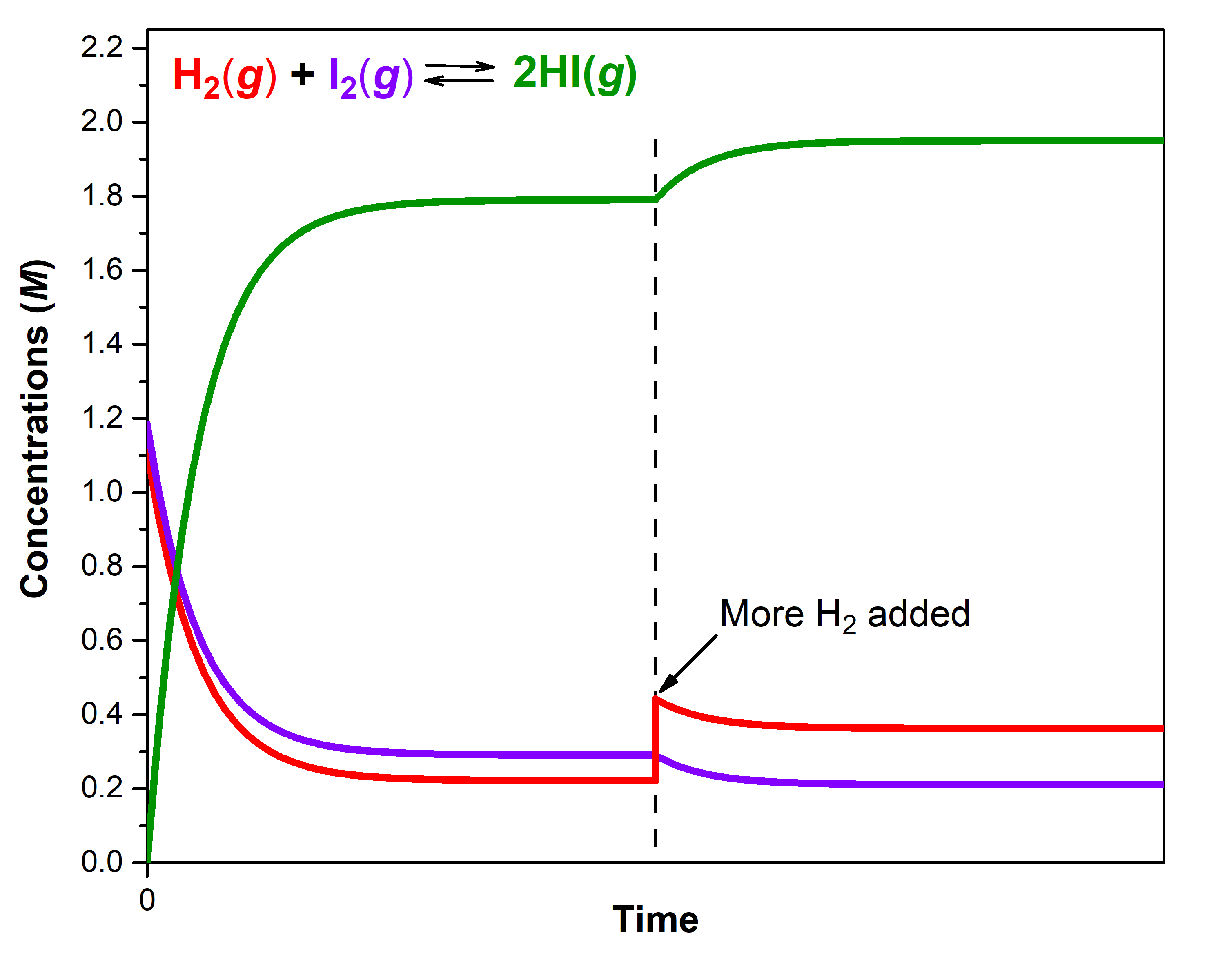
A mixture of gases at 400 °C with [H2] = 0.221 M, [I2] = 0.290 M, and [HI] = 1.790 M is at equilibrium in a closed container. For this mixture, Qc = Kc = 50.0.
If additional H2(g) is introduced into the container quickly such that [H2] doubles before it begins to react (that is, the new [H2]t = 0.442 M), Qc is now ½ of Kc:
The reaction will proceed in the forward direction (towards products) to reach a new equilibrium. You can use an ICE table and calculate that the new equilibrium concentrations are [H2] = 0.362 M, [I2] = 0.210 M, and [HI] = 1.950 M.
Notice that [H2]new equilibrium (0.362 M) is less than the doubled concentration (0.442 M) but more than [H2]first equilibrium (0.221 M). The equilibrium has shifted to partially counteract the change in H2 concentration. Because of the shift, the concentration of the other reactant decreases and the concentration of the product increases. To verify that these new concentrations are equilibrium concentrations, calculate Q:
The figure below illustrates graphically the effect of adding H2 to the reaction that was at equilibrium.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

