Organic Nomenclature, Basic
Alkanes
The International Union of Pure and Applied Chemistry (IUPAC) has devised a system of nomenclature that begins with the names of the alkanes and can be adjusted from there to account for more complicated structures. The nomenclature for alkanes is based on two rules:
- To name an alkane, first identify the longest chain of carbon atoms in its structure. A two-carbon chain is called ethane; a three-carbon chain, propane; and a four-carbon chain, butane. Longer chains are named as follows: pentane (five-carbon chain), hexane (6), heptane (7), octane (8), nonane (9), and decane (10).
- Add prefixes to the name of the longest chain to indicate the positions and names of substituents. Substituents are branches or functional groups that replace hydrogen atoms on a chain. The position of a substituent or branch is identified by the number of the carbon atom it is bonded to in the chain. We number the carbon atoms in the chain by counting from the end of the chain nearest the substituents. Multiple substituents are named individually and placed in alphabetical order at the front of the name.
When more than one substituent is present, either on the same carbon atom or on different carbon atoms, the substituents are listed alphabetically. Because the carbon atom numbering begins at the end closest to a substituent, the longest chain of carbon atoms is numbered in such a way as to produce the lowest number for the substituents. The ending -o replaces -ide at the end of the name of an electronegative substituent (in ionic compounds, the negatively charged ion ends with -ide like chloride; in organic compounds, such atoms are treated as substituents and the -o ending is used). The number of substituents of the same type is indicated by the prefixes di- (two), tri- (three), tetra- (four), and so on (for example, difluoro- indicates two fluoride substituents).
Example 1
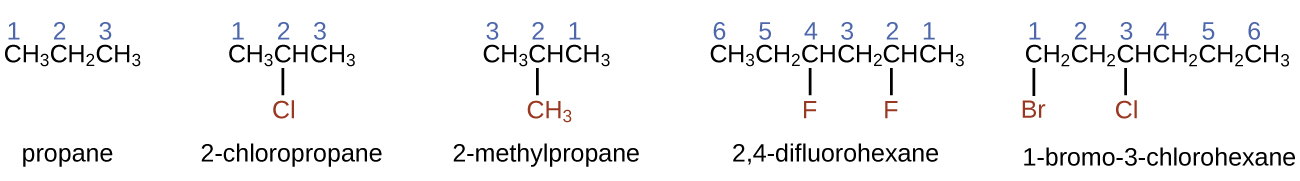
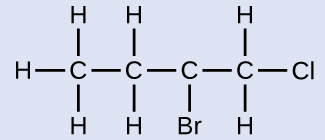
Naming Halogen-substituted AlkanesName the molecule whose structure is shown here:
Solution
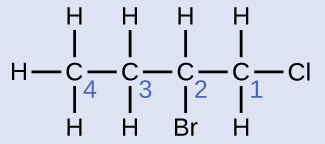
The four-carbon chain is numbered from the end with the chlorine atom. This puts the substituents on positions 1 and 2 (numbering from the other end would put the substituents on positions 3 and 4). Four carbon atoms means that the base name of this compound will be butane. The bromine at position 2 will be described by adding 2-bromo-; this will come at the beginning of the name, since bromo- comes before chloro- alphabetically. The chlorine at position 1 will be described by adding 1-chloro-, resulting in the name of the molecule being 2-bromo-1-chlorobutane.
Check Your Learning
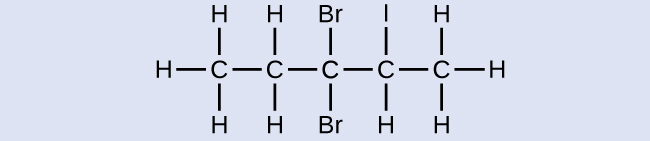
Name the following molecule:
Answer:
3,3-dibromo-2-iodopentane
We call a substituent that contains one less hydrogen than the corresponding alkane an alkyl group. The name of an alkyl group is obtained by dropping the suffix -ane of the alkane name and adding -yl:
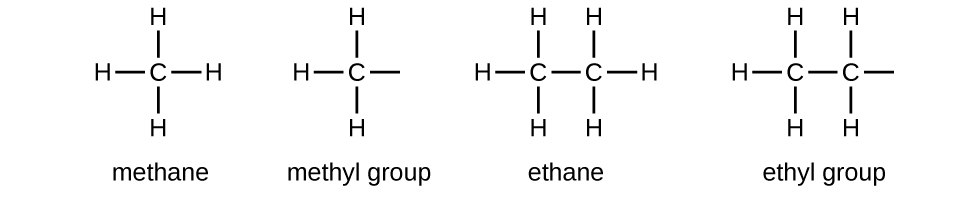
The open bonds in the methyl and ethyl groups indicate that these alkyl groups are bonded to another atom.
Example 2
Naming Substituted Alkanes
Name the molecule whose structure is shown here:
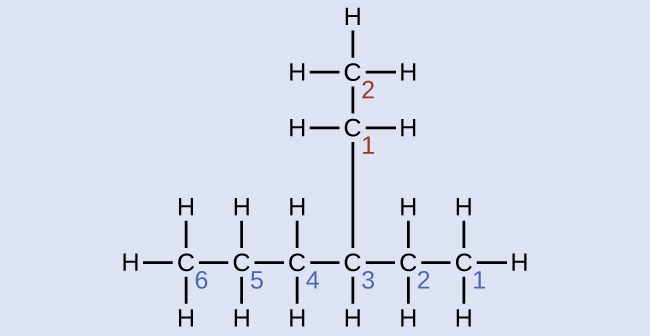
Solution
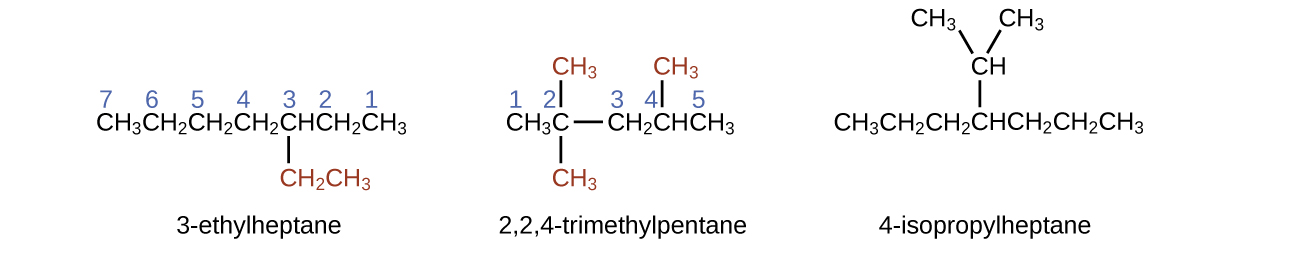
The longest carbon chain runs horizontally across the page and contains six carbon atoms (this makes the base of the name hexane, but we will also need to incorporate the name of the branch). In this case, we want to number from right to left (as shown by the blue numbers) so the branch is connected to carbon 3 (imagine the numbers from left to right—this would put the branch on carbon 4, violating our rules). The branch attached to position 3 of our chain contains two carbon atoms (numbered in red)—so we take our name for two carbons eth- and attach -yl at the end to signify we are describing a branch. Putting all the pieces together, this molecule is 3-ethylhexane.
Check Your Learning
Name the following molecule:
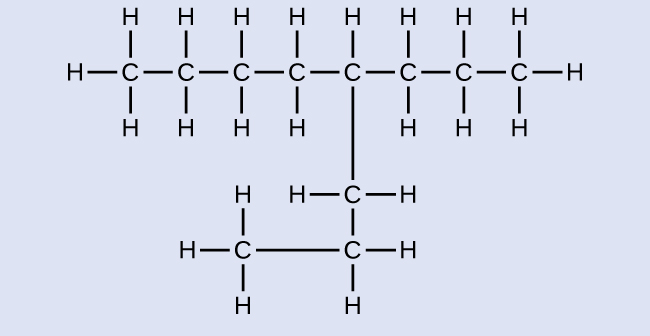
Answer:
4-propyloctane<
Some hydrocarbons can form more than one type of alkyl group when the hydrogen atoms that would be removed have different “environments” in the molecule. This diversity of possible alkyl groups can be identified in the following way: The four hydrogen atoms in a methane molecule are equivalent; they all have the same environment. They are equivalent because each is bonded to a carbon atom (the same carbon atom) that is bonded to three hydrogen atoms. Removal of any one of the four hydrogen atoms from methane forms a methyl group. Likewise, the six hydrogen atoms in ethane are equivalent and removing any one of these hydrogen atoms produces an ethyl group. Each of the six hydrogen atoms is bonded to a carbon atom that is bonded to two other hydrogen atoms and a carbon atom. However, in both propane and 2–methylpropane, there are hydrogen atoms in two different environments, distinguished by the adjacent atoms or groups of atoms:
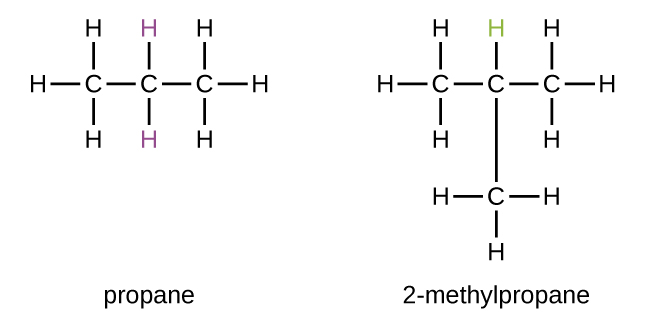
Each of the six equivalent hydrogen atoms of the first type in propane and each of the nine equivalent hydrogen atoms of that type in 2-methylpropane (all shown in black) are bonded to a carbon atom that is bonded to only one other carbon atom. The two purple hydrogen atoms in propane are of a second type. They differ from the six hydrogen atoms of the first type in that they are bonded to a carbon atom bonded to two other carbon atoms. The green hydrogen atom in 2-methylpropane differs from the other nine hydrogen atoms in that molecule and from the purple hydrogen atoms in propane. The green hydrogen atom in 2-methylpropane is bonded to a carbon atom bonded to three other carbon atoms. Two different alkyl groups can be formed from each of these molecules, depending on which hydrogen atom is removed. The names and structures of these and several other alkyl groups are listed in Figure 1.
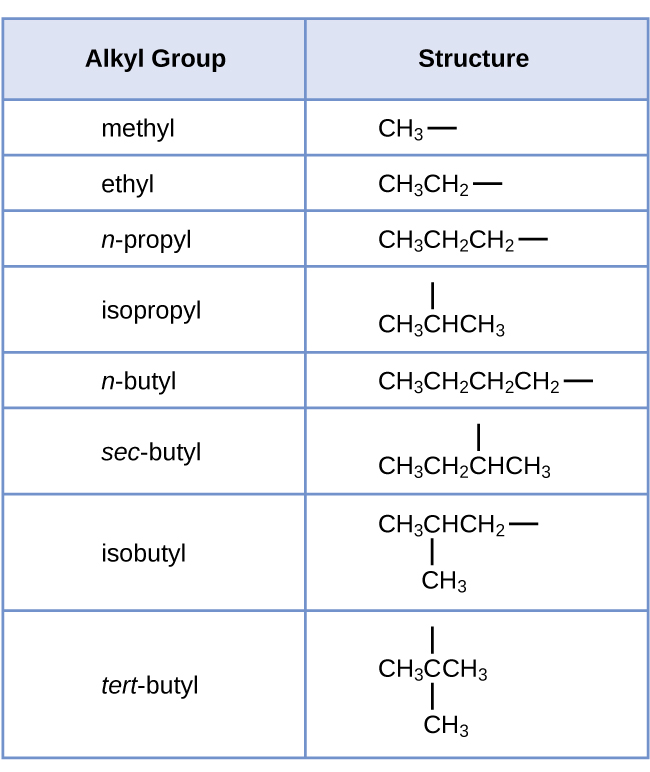
Note that alkyl groups do not exist as stable independent entities. They are always a part of some larger molecule. The location of an alkyl group on a hydrocarbon chain is indicated in the same way as any other substituent:
Alcohols
The name of an alcohol comes from the hydrocarbon from which it was derived. The final -e in the name of the hydrocarbon is replaced by -ol, and the carbon atom to which the –OH group is bonded is indicated by a number placed before the name.
Consider the following example:
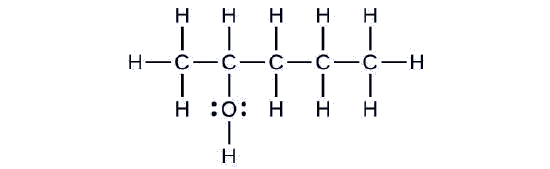
The carbon chain contains five carbon atoms. If the hydroxyl group was not present, we would have named this molecule pentane. To address the fact that the hydroxyl group is present, we change the ending of the name to -ol. In this case, since the –OH is attached to carbon 2 in the chain, we would name this molecule 2-pentanol.
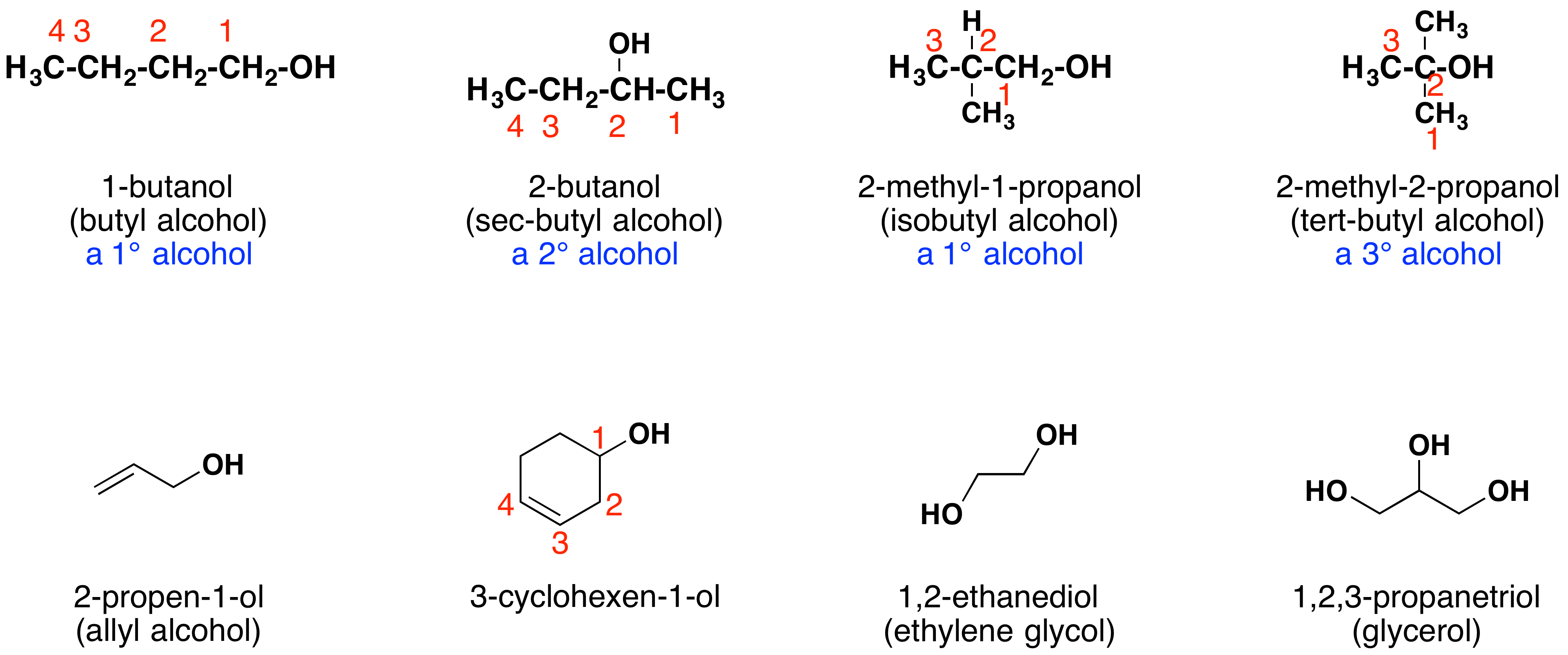
Other examples of IUPAC nomenclature are shown below, together with the common names often used for some of the simpler compounds. For the mono-functional alcohols, this common system consists of naming the alkyl group followed by the word alcohol. Alcohols may also be classified as primary, 1º, secondary, 2º, and tertiary, 3º. This terminology refers to alkyl substitution of the carbon atom bearing the hydroxyl group (colored blue in the illustration).
Carboxylic Acids
The –e ending is removed from the name of the parent carbon chain and is replaced -anoic acid. Since a carboxylic acid group must always lie at the end of a carbon chain, it is always is given the #1 location position in numbering and it is not necessary to include it in the name. Many carboxylic acids are called by the common names that were chosen by chemists to usually describe the origin of the compound.
| Formula | Common Name | Source | IUPAC Name |
|---|---|---|---|
| HCOOH | formic acid | ants (L. formica) | methanoic acid |
| CH3COOH | acetic acid | vinegar (L. acetum) | ethanoic acid |
| CH3CH2COOH | propionic acid | milk (Gk. protus prion) | propanoic acid |
| CH3(CH2)2COOH | butyric acid | butter (L. butyrum) | butanoic acid |
| CH3(CH2)3COOH | valeric acid | valerian root | pentanoic acid |
| CH3(CH2)4COOH | caproic acid | goats (L. caper) | hexanoic acid |
| CH3(CH2)5COOH | enanthic acid | vines (Gk. oenanthe) | heptanoic acid |
| CH3(CH2)6COOH | caprylic acid | goats (L. caper) | octanoic acid |
| CH3(CH2)7COOH | pelargonic acid | pelargonium (an herb) | nonanoic acid |
| CH3(CH2)8COOH | capric acid | goats (L. caper) | decanoic acid |
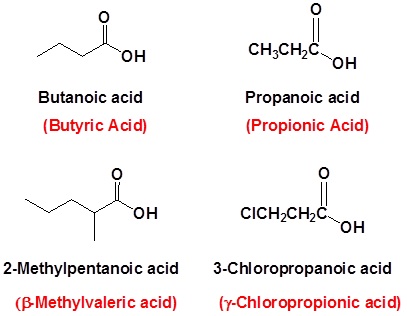
When a carboxyl group is added to a ring the suffix -carboxylic acid is added to the name of the cyclic compound. The ring carbon attached to the carboxyl group is given the #1 location number.
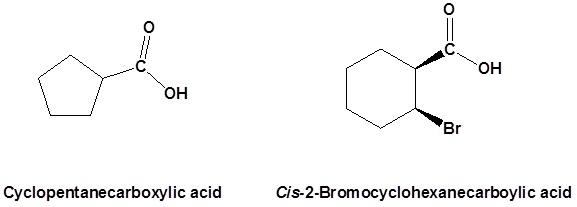
Salts of carboxylic acids are named by writing the name of the cation followed by the name of the acid with the –ic acid ending replaced by an –ate ending. This is true for both the IUPAC and Common nomenclature systems.
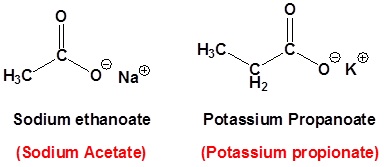
For dicarboxylic acids the location numbers for both carboxyl groups are omitted because both functional groups are expected to occupy the ends of the parent chain. The ending –dioic acid is added to the end of the parent chain.
Esters
Esters are named as if the alkyl chain from the alcohol is a substituent. No number is assigned to this alkyl chain. This is followed by the name of the parent chain from the carboxylic acid part of the ester with an –e remove and replaced with the ending –oate.
For example:
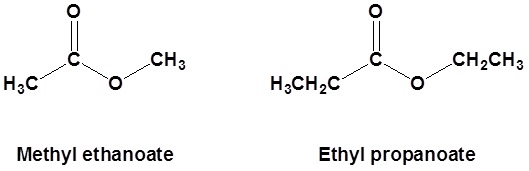
- First, identify the oxygen that is part of the continuous chain and bonded to carbon on both sides. (On one side of this oxygen there will be a carbonyl present but on the other side there won’t be.)
- Second, begin numbering the carbon chains on either side of the oxygen identified in step 1.
- Next, use this format: [alkyl on side further from the carbonyl] (space) [alkane on the side with the carbonyl]
- Finally, change the ending of the alkane on the same side as the carbonyl from -e to -oate.
- When an ester group is attached to a ring, the ester is named as a substituent on the ring.
In both common and International Union of Pure and Applied Chemistry (IUPAC) nomenclature, the –ic ending of the parent acid is replaced by the suffix –ate.
| Condensed Structural Formula | Common Name | IUPAC Name |
|---|---|---|
| HCOOCH3 | methyl formate | methyl methanoate |
| CH3COOCH3 | methyl acetate | methyl ethanoate |
| CH3COOCH2CH3 | ethyl acetate | ethyl ethanoate |
| CH3CH2COOCH2CH3 | ethyl propionate | ethyl propanoate |
| CH3CH2CH2COOCH(CH3)2 | isopropyl butyrate | isopropyl butanoate |
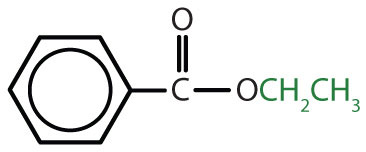 |
ethyl benzoate | ethyl benzoate |
Amines
The word “amine” is derived from ammonia, and the class of compounds known as amines therefore are commonly named as substituted ammonias. In this system, primary amines (RNH2), having only one substituent on nitrogen, are named with the substituent as a prefix. More systematic nomenclature appends -amine to the longest chain, as for alcohols:
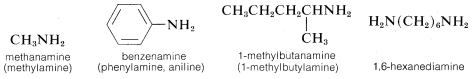
Secondary (R2NH) and tertiary amines (R3N), which have two and three substituents on nitrogen, commonly are named as N-substituted amines. N is included to indicate that the substituent is on the nitrogen atom unless there is no ambiguity as to where the substituent is located. Each of the R groups is named as a separate word, except when the groups are identical, in which case the prefix di or bis may be used (di is used for simple groups, bis for substituted groups):
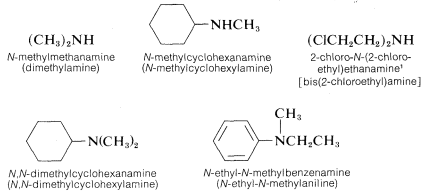
The nomenclature of amines is complicated by the fact that several different nomenclature systems exist. The four compounds shown in the top row of the following diagram are all C4H11N isomers.
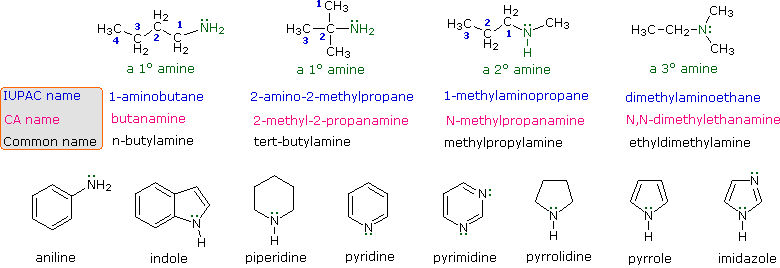
- The IUPAC names are listed first and colored blue. This system names amine functions as substituents on the largest alkyl group. The simple -NH substituent found in 1º-amines is called an amino group. For 2º and 3º-amines a compound prefix (e.g. dimethylamino in the fourth example) includes the names of all but the root alkyl group.
- The Chemical Abstract Service has adopted a nomenclature system in which the suffix -amine is attached to the root alkyl name. For 1º-amines such as butanamine (first example) this is analogous to IUPAC alcohol nomenclature (-ol suffix). The additional nitrogen substituents in 2º and 3º-amines are designated by the prefix N- before the group name. These CA names are colored magenta in the diagram.
- Finally, a common system for simple amines names each alkyl substituent on nitrogen in alphabetical order, followed by the suffix -amine. These are the names given in the last row (colored black).
Many aromatic and heterocyclic amines are known by unique common names, the origins of which are often unknown to the chemists that use them frequently since these names are not based on a rational system.
Amides
Primary amides are named by changing the name of the acid by dropping the -oic acid or -ic acid endings and adding -amide. The carbonyl carbon is given the #1 location number. It is not necessary to include the location number in the name because it is assumed that the functional group will be on the end of the parent chain. For example, the following three amides are called methanamide or formamide, ethanamide or acetamide, and benzamide.
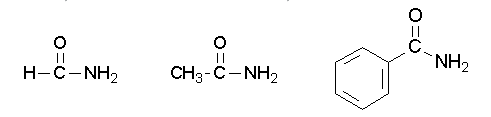
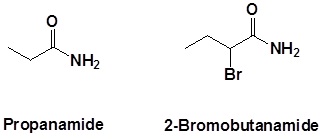
Further examples are shown below:
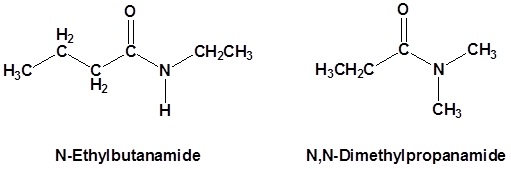
Secondary amides are named by using an upper case N to designate that the alkyl group is on the nitrogen atom. Alkyl groups attached to the nitrogen are named as substituents. The letter N is used to indicate they are attached to the nitrogen. For example, the molecule below is called N-methylpropanamide:
![]()
Tertiary amides are named in the same way as secondary amides, but with two N’s
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

