D12.6 Stereoisomers: Geometric Isomers
Stereoisomers are molecules that have the same molecular formula and the same atomic connectivity, but differ in the orientation of the atoms in 3D space. Hence, molecules that are stereoisomers of each other stem from the same structural isomer. There are several different types of stereoisomers. First we will discuss geometric isomers.
The two carbon atoms in a C=C double bond cannot freely rotate with respect to each other because such rotation would break the π bond. Once the π bond is broken, there would be free rotation around the remaining σ bond, but an input of ~250 kJ/mol is needed first to break the π bond. This rigid characteristic of a C=C double bond gives rise to geometric isomers, stereoisomers that differ in the orientation of the groups connected to the two carbons in a C=C bond.
For example, there are two geometric isomers of 2-butene, cis-2-butene and trans-2-butene:

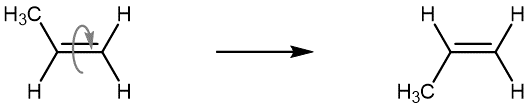
The isomer with both methyl groups on the same side of the double bond is called a cis isomer (above, the methyl groups are shown as both being above the double bond). The one with the methyl groups on opposite sides is called a trans isomer. Geometric isomers have different physical properties, such as boiling point. Because they will not readily convert from one to another, they are different substances, and in nomenclature, they are distinguished by the “cis” and “trans” prefix. The figure below shows an animation of a cis-2-butene molecule’s various conformations that are accessible at room temperature.
Cis-trans isomerism is only possible when there are two different groups at each end of a double bond. In other words, in a generic alkene molecule:
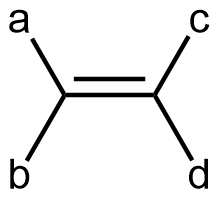
there can be geometric isomers if ‘a’ and ‘b’ are different groups and ‘c’ and ‘d’ are different groups. If both groups on either end of the double bond were the same (‘a’ the same as ‘b’, or, ‘c’ the same as ‘d’), there is no cis and trans isomers (rotating around the double bond would give the same molecular structure). For example, if rotation about the C=C bond were to occur in a propene molecule, the result would be the same propene molecule (just viewed from a different perspective).
Exercise: Isomers
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂

